- Department of Neurosurgery, Medical Faculty - Mataram University General Province West Nusa Tenggara Hospitals, Mataram City, West Nusa Tenggara, Indonesia
- Department of Neurosurgery, Dr. Soeradji Tirtonegoro Central Public Hospital, Klaten, Central Java, Indonesia
- Department of Neurosurgery, Faculty of Medicine, Mataram University, Mataram, West Nusa Tenggara, Indonesia
- Department of Neurosurgery, Udayana University Hospital, Medical Faculty of Udayana University, Denpasar, Indonesia
- Research Unit, Faculty of Medicine, Al Azhar Islamic University, Mataram, West Nusa Tenggara, Indonesia
- Department of Neurosurgery, Faculty of Medicine and Health Sciences, Muhammadiyah Makassar University/Medical Faculty of Hasanudin University, Makassar, South Sulawesi, Indonesia
Correspondence Address:
Rohadi Muhammad Rosyidi, Department of Neurosurgery, Medical Faculty - Mataram University General Province West Nusa Tenggara Hospitals, Mataram City, West Nusa Tenggara, Indonesia.
DOI:10.25259/SNI_176_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Rohadi Muhammad Rosyidi1, Hanan Anwar Rusidi2, Januarman Januarman3, Bambang Priyanto1, Dewa Putu Wisnu Wardhana4, Rozikin Rozikin5, Wahyudi Wahyudi6, Wisnu Baskoro2. Centella asiatica effect on traumatic brain injury: A systematic review. 19-Jul-2024;15:248
How to cite this URL: Rohadi Muhammad Rosyidi1, Hanan Anwar Rusidi2, Januarman Januarman3, Bambang Priyanto1, Dewa Putu Wisnu Wardhana4, Rozikin Rozikin5, Wahyudi Wahyudi6, Wisnu Baskoro2. Centella asiatica effect on traumatic brain injury: A systematic review. 19-Jul-2024;15:248. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12998
Abstract
Background: Mortality and morbidity in traumatic brain injury (TBI) cases remain a global problem. Various therapeutic modalities have been researched, including using herbal medicine. Centella asiatica has a lot of potential in neuropharmacology for various diseases. This systematic review aims to comprehensively review the currently available data about the impact of C. asiatica on TBI in a rat model.
Methods: Systematic searches were conducted on PubMed, Scopus, and Google Scholar up to July 2023. This study follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses protocol. Researchers screened the titles and abstracts of all identified studies and then selected relevant studies through full-text reviews. Studies reported the effect of C. asiatica on animal model of TBI were included in the study. Data were extracted, and the result was reported using descriptive analysis. The risk of bias was evaluated using SYRCLE.
Results: Four studies met the inclusion criteria. One study highlighted the potential neuroprotective effects of Asiatic acid, one study explored spade leaf extract phytosome, while the rest used C. asiatica extracts. The primary findings of the included research revealed that C. asiatica might reduce oxidative stress, decrease neuronal apoptosis, have anti-inflammatory properties, alleviate neurological dysfunction, reduce cerebral edema, and boost cognitive performance in the TBI-induced rat’s model.
Conclusion: This review suggests that C. asiatica had the potential to benefit the TBI-induced rat model in terms of decreasing morbidity. Nevertheless, more studies are needed to perform a meta-analysis and ascertain the effects of C. asiatica on TBI in animal models.
Keywords: Centella asiatica, Brain injury, Rats model
INTRODUCTION
Traumatic brain injury (TBI) remains a significant global health issue, characterized by high rates of mortality and morbidity worldwide. Annually, the incidence of TBI is estimated to reach 50 million cases.[
The pathophysiology of TBI involves various mechanisms resulting in brain injury, which can be categorized into primary and secondary injuries. Primary injury occurs immediately following a direct force impact, while secondary injuries develop subsequent to the initial impact.[
No complete and effective treatment modalities currently exist for secondary injuries in TBI, which involve complex pathophysiology. Over the past few decades, extensive research has been conducted into various therapeutic approaches for TBI. Among these, the use of herbs as a complementary therapy has garnered significant attention.
Centella asiatica is a tropical also known as Asiatic pennywort, Indian pennywort, wild violet, tiger herb, Indian water navelwort, gotu kola, and pegagan, which is a vine herb that is widely used and cultivated as a medicinal plant in Asia.[
C. asiatica has extensive pharmacological potential. Recent studies report C. asiatica to have roles in several conditions, such as reducing oxidative stress, antipyretic, antidepressant, anticonvulsant, anxiolytic, anticancer, anti-infective, anti-wrinkle, wound-healing, anti-inflammatory properties, and neuroprotective.[
Despite its wide use in various cases, the benefits of neuropharmacological use of C. asiatica in TBI are still receiving less attention. The clinical impact of C. asiatica on TBI has been researched through a number of randomized controlled trials. However, no studies summarize the evidence on the effects of C. asiatica on TBI and its associated potency. This study will thoroughly analyze all available data to verify the impact of C. asiatica on TBI and its related potency.
MATERIALS AND METHODS
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses were used to conduct this systematic review. In addition, this systematic review follows the population, intervention, comparison, and outcome specification.
Search strategy
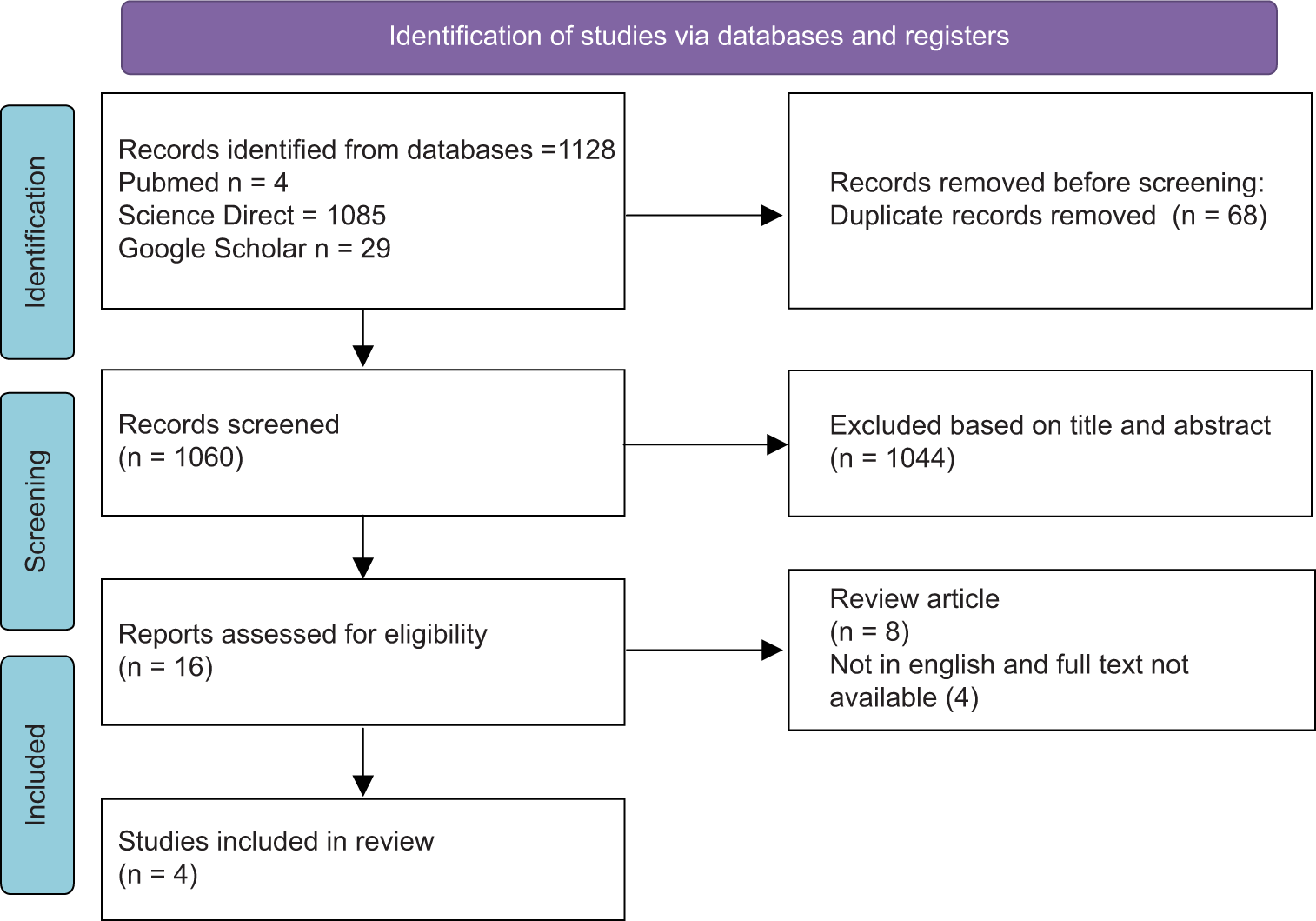
Several electronic databases were used in an electronic search for original publications from the beginning until July 2023, including PubMed, Science Direct, and Google Scholar. Literature search utilized keywords and filters in the form of Medical Subject Headings and text terms. The filters used were “Centella” AND “Brain Injury, Traumatic.” Boolean operators (OR/AND) were used to combine words during the process, as shown in
Study selection
Studies that primarily discuss the effect of C. asiatica on TBI are included under the inclusion criteria. The review considers various study types, including experimental research such as clinical trials and randomized controlled trials using animal models, and included studies must prominently feature C. asiatica extract as the primary intervention and report relevant outcomes.
Conversely, exclusion criteria are equally crucial. Studies unrelated to the effects of C. asiatica on TBI will be excluded. Not in English, articles, reviews, letters, abstracts, or editorial papers will also be omitted.
Data extraction
A typical data extraction form was used for the data extraction process. Data extracted from each selected study included the main author, publication year, follow-up period, control group, and outcome in TBI.
Quality assessment
The systematic review authors used the SYRCLE’s risk of bias tool to assess the quality of the included animal studies.[
RESULTS
Out of 1128 identified articles, 68 were excluded due to duplication. After the duplicated articles were excluded, 1060 articles were screened. Only four studies met the inclusion criteria and were included in this study after 16 full-text articles were read, as shown in
Only four articles out of the more than a thousand initially found publications passed the requirements for inclusion in this systematic review [
The study conducted by Han et al.[
Nafiisah et al.[
In a rat model of TBI, C. asiatica’s impact on serum tumor necrosis factor- levels were examined in a study by Nafiisah et al.[
Assessment of risk of bias of included studies
The SYRCLE’s risk of bias tool is a checklist that evaluates the methodological quality of animal studies.[
DISCUSSION
Research summarizing the role of C. asiatica in TBI has not been conducted to our knowledge. The study included four articles that fulfilled the eligibility criteria that had been set. In this study, we did not conduct a meta-analysis due to the limited data required. All four studies involved were preclinical studies using TBI-induced rat models that evaluated C. asiatica as a treatment modality for TBI.
The processes that occur in TBI include primary and secondary injuries. Damage and distortion to brain structures due to mechanical processes at the onset of trauma is known as primary brain injury.[
After the initial trauma, secondary brain injury can happen hours, days, or even months later[
Herbal plant research is currently prevalent throughout the globe. C. asiatica is one of the many botanical plants utilized in research. C. asiatica belongs to the family Umbelliferae.[
Cellular damage in secondary brain injury is primarily due to oxidative stress processes that produce reactive oxygen species.[
C. asiatica also plays a role in the protection against neuronal cell apoptosis. In secondary brain injury, neuronal cell apoptosis occurs due to the activation of enzymes involved in cell apoptosis, such as caspases and calpain, by various biochemical signals.[
The role of C. asiatica as an anti-inflammatory was reported by the study of Nafisah et al.,[
TBI causes a decrease in phospholipids, and this condition can last for an extended period. Phospholipids are membrane constituents and play a role in re-myelination.[
Limitations of the review
The systematic review of the effect of C. asiatica on TBI has several limitations that should be considered when interpreting the findings. First, the review included only four studies that met the inclusion criteria, which may not provide a comprehensive overview of the effects of C. asiatica on TBI. Second, the included studies varied in terms of study design, sample size, duration of intervention, and outcome measures, which may limit the ability to draw definitive conclusions about the effects of C. asiatica on TBI. Third, the included studies have a high risk of bias, which may affect the validity of the review’s findings. Fourth, the included studies used different dosages and formulations of C. asiatica, which may affect the consistency of the results. Fifth, the studies involved are limited to animal models; human studies are needed for high-quality studies. Finally, the review was conducted primarily in English, which may have resulted in language bias. Relevant studies published in other languages may have been missed, which may affect the comprehensiveness of the review.
CONCLUSION
While the systematic review provides evidence suggesting that C. asiatica may have a positive effect on TBI, the study’s limitations should be considered when interpreting the findings. Additional high-quality research is necessary to confirm the effects of C. asiatica on TBI and determine the optimal dosage and formulation of C. asiatica for treating TBI.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Akamatsu Y, Hanafy KA. Cell death and recovery in traumatic brain injury. Neurotherapeutics. 2020. 17: 446-56
2. Bandopadhyay S, Mandal S, Ghorai M, Jha NK, Kumar M, Radha GA. Therapeutic properties and pharmacological activities of asiaticoside and madecassoside: A review. J Cell Mol Med. 2023. 27: 593-608
3. Bansal K, Bhati H, Bajpai M. Recent insights into therapeutic potential and nanostructured carrier systems of Centella asiatica An evidence-based review. Pharmacol Res Modern Chin Med. 2024. 10: 100403
4. Capizzi A, Woo J, Verduzco-Gutierrez M. Traumatic brain injury: An overview of epidemiology, pathophysiology, and medical management. Med Clin N Am. 2020. 104: 213-38
5. Castelnovo LF, Bonalume V, Melfi S, Ballabio M, Colleoni D, Magnaghi V. Schwann cell development, maturation and regeneration: A focus on classic and emerging intracellular signaling pathways. Neural Regen Res. 2017. 12: 1013-23
6. Chandrakesan A, Muruhan S, Sayanam RR. Morin inhibiting photocarcinogenesis by targeting ultraviolet-B-induced oxidative stress and inflammatory cytokines expression in swiss albino mice. Int J Nutr Pharmacol Neurol Dis. 2018. 8: 41-6
7. Chen D, Zhang XY, Sun J, Cong QJ, Chen WX, Ahsan HM. Asiatic acid protects dopaminergic neurons from neuroinflammation by suppressing mitochondrial ros production. Biomol Ther. 2019. 27: 442-9
8. Damkerngsuntorn W, Rerknimitr P, Panchaprateep R, Tangkijngamvong N, Kumtornrut C, Kerr SJ. The effects of a standardized extract of Centella asiatica on postlaser resurfacing wound healing on the face: A split-face, double-blind, randomized, placebo-controlled trial. J Altern Complement Med. 2020. 26: 529-36
9. Ding L, Liu T, Ma J. Neuroprotective mechanisms of Asiatic acid. Heliyon. 2023. 9: e15853
10. Diniz LR, Calado LL, Duarte AB, de Sousa DP. Centella asiatica and its metabolite asiatic acid: Wound healing effects and therapeutic potential. Metabolites. 2023. 13: 276
11. Dunne J, Quiñones-Ossa GA, Still EG, Suarez MN, GonzálezSoto JA, Vera DS. The epidemiology of traumatic brain injury due to traffic accidents in Latin America: A narrative review. J Neurosci Rural Pract. 2020. 11: 287-90
12. Fesharaki-Zadeh A, Datta D. An overview of preclinical models of traumatic brain injury (TBI): Relevance to pathophysiological mechanisms. Front Cell Neurosci. 2024. 18: 1371213
13. Fesharaki-Zadeh A. Oxidative stress in traumatic brain injury. Int J Mol Sci. 2022. 23: 13000
14. Freire MA, Rocha GS, Bittencourt LO, Falcao D, Lima RR, Cavalcanti JR. Cellular and molecular pathophysiology of traumatic brain injury: What have we learned so far?. Biology. 2023. 12: 1139
15. Galgano M, Toshkezi G, Qiu X, Russell T, Chin L, Zhao LR. Traumatic brain injury: Current treatment strategies and future endeavors. Cell Transplant. 2017. 26: 1118-30
16. Gray NE, Alcazar Magana A, Lak P, Wright KM, Quinn J, Stevens JF. Centella asiaticaphytochemistry and mechanisms of neuroprotection and cognitive enhancement. Phytochem Rev. 2018. 17: 161-94
17. Gray NE, Hack W, Brandes MS, Zweig JA, Yang L, Marney L. Amelioration of age-related cognitive decline and anxiety in mice by Centella asiatica extract varies by sex, dose and mode of administration. Front Aging. 2024. 5: 1357922
18. Han F, Yan N, Huo J, Chen X, Fei Z, Li X. Asiatic acid attenuates traumatic brain injury via upregulating Nrf2 and HO-1 expression. Int J Clin Exp Med. 2018. 11: 360-6
19. Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014. 14: 43
20. Jahan R, Hossain S, Seraj S, Nasrin D, Khatun Z, Das PR. Centella asiatica (L.) Urb.: Ethnomedicinal uses and their scientific validations. Am Eurasian J Sustain Agric. 2012. 6: 261-70
21. Jazmi AF, Alfiantya PF, Nurarifah SA, Purmitasari EA, Vitania LA, Riawan W. Spade leaf extract phytosome modulates KROX-20, neuregulin-1, phospholipids, and cognitive function of traumatic brain injury model in rats. Indones J Cancer Chemoprev. 2017. 6: 105
22. Kamran S, Sinniah A, Abdulghani MA, Alshawsh MA. Therapeutic potential of certain terpenoids as anticancer agents: A scoping review. Cancers. 2022. 14: 1100
23. Kandasamy A, Aruchamy K, Rangasamy P, Varadhaiyan D, Gowri C, Oh TH. Phytochemical analysis and antioxidant activity of Centella asiatica extracts: An experimental and theoretical investigation of flavonoids. Plants (Basel, Switzerland). 2023. 12: 3547
24. Kinoshita K. Traumatic brain injury: Pathophysiology for neurocritical care. J Intensive Care. 2016. 4: 29
25. Krishnamurthy RG, Senut MC, Zemke D, Min J, Frenkel MB, Greenberg EJ. Asiatic acid, a pentacyclic triterpene from Centella asiatica is neuroprotective in a mouse model of focal cerebral ischemia. J Neurosci Res. 2009. 87: 2541-50
26. Lokanathan Y, Omar N, Ahmad Puzi NN, Saim A, Hj Idrus R. Recent updates in neuroprotective and neuroregenerative potential of Centella asiatica. Malays J Med Sci. 2016. 23: 4-14
27. Lotocki G, de Rivero Vaccari JP, Alonso O, Molano JS, Nixon R, Safavi P. Oligodendrocyte vulnerability following traumatic brain injury in rats. Neurosci Lett. 2011. 499: 143-8
28. Luo Y, Yang YP, Liu J, Li WH, Yang J, Sui X. Neuroprotective effects of madecassoside against focal cerebral ischemia reperfusion injury in rats. Brain Res. 2014. 1565: 37-47
29. Matthews DG, Caruso M, Murchison CF, Zhu JY, Wright KM, Harris CJ. Centella asiatica improves memory and promotes antioxidative signaling in 5XFAD mice. Antioxidants (Basel, Switzerland). 2019. 8: 630
30. Nafiisah N, Faniyah F, Pratama YM. Anti-inflammatory effect of Centella asiatica (L.) extract by decreasing TNF-α serum levels in rat model of traumatic brain injury. Maj Kedokt Bandung. 2021. 53: 63-6
31. Nafiisah N, Prihatno MM, Novrial D. Effect of Centella asiatica L. extract on apoptosis and Bcl-2 immunoexpression of pyramidal cells in traumatic brain injury rat model. Int J Nutr Pharmacol Neurol Dis. 2021. 11: 242-8
32. Ng SY, Lee AY. Traumatic brain injuries: Pathophysiology and potential therapeutic targets. Front Cell Neurosci. 2019. 13: 528
33. Nguyen A, Patel AB, Kioutchoukova IP, Diaz MJ, Lucke-Wold B. Mechanisms of mitochondrial oxidative stress in brain injury: From pathophysiology to therapeutics. Oxygen. 2023. 3: 163-78
34. Park JH, Choi JY, Son DJ, Park EK, Song MJ, Hellström M. Anti-inflammatory effect of titrated extract of Centella asiatica in phthalic anhydride-induced allergic dermatitis animal model. Int J Mol Sci. 2017. 18: 738
35. Qian S, Wei Z, Yang W, Huang J, Yang Y, Wang J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front Oncol. 2022. 12: 985363
36. Rana A, Singh S, Sharma R, Kumar A. Traumatic brain injury altered normal brain signaling pathways: Implications for novel therapeutics approaches. Curr Neuropharmacol. 2019. 17: 614-29
37. Rauchman SH, Albert J, Pinkhasov A, Reiss AB. Mild-to-moderate traumatic brain injury: A review with focus on the visual system. Neurol Int. 2022. 14: 453-70
38. Sun B, Wu L, Wu Y, Zhang C, Qin L, Hayashi M. Therapeutic potential of Centella asiatica and its triterpenes: A review. Front Pharmacol. 2020. 11: 568032
39. Thau-Zuchman O, Gomes RN, Dyall SC, Davies M, Priestley JV, Groenendijk M. Brain phospholipid precursors administered post-injury reduce tissue damage and improve neurological outcome in experimental traumatic brain injury. J Neurotrauma. 2019. 36: 25-42
40. Tolescu RŞ, Zorilă MV, Şerbănescu MS, Kamal KC, Zorilă GL, Dumitru I. Severe traumatic brain injury (TBI)-a seven-year comparative study in a Department of Forensic Medicine. Rom J Morphol Embryol. 2020. 61: 95-103
41. Wong JH, Barron AM, Abdullah JM. Mitoprotective effects of Centella asiatica (L.) Urb.: Anti-inflammatory and neuroprotective opportunities in neurodegenerative disease. Front Pharmacol. 2021. 12: 687935