- Department of Neurosurgery, Sakra World Hospital, Bengaluru, Karnataka, India
- Department of Neurosurgery, San Fernando Hospital, San Fernando, Argentina
- Department of Neurosurgery, Institute de Seguridad y Servicios Sociales de los Trabajadores Del Estrado, Mexico City, Mexico
- Department of Neurosurgery, Neurosurgery Clinic,Birgunj,Nepal.
Correspondence Address:
Bipin Chaurasia, Department of Neurosurgery, Neurosurgery Clinic, Birgunj, Nepal.
DOI:10.25259/SNI_14_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mohammad Mazhar Khan1, Abinash Dutta1, Deepak Rajappa1, Dattatraya Mallik1, Matias Baldoncini2, Carlos Castillo Rangel3, Bipin Chaurasia4. Facial nerve electrical motor evoked potential in cerebellopontine angle tumors for its anatomical and functional preservation. 31-May-2024;15:182
How to cite this URL: Mohammad Mazhar Khan1, Abinash Dutta1, Deepak Rajappa1, Dattatraya Mallik1, Matias Baldoncini2, Carlos Castillo Rangel3, Bipin Chaurasia4. Facial nerve electrical motor evoked potential in cerebellopontine angle tumors for its anatomical and functional preservation. 31-May-2024;15:182. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12917
Abstract
Background: Among the technical measures to preserve facial nerve (FN) function, intraoperative neuromonitoring has become mandatory and is constantly being scrutinized. Hence, to determine the efficacy of FN motor evoked potentials (FNMEPs) in predicting long-term motor FN function following cerebellopontine angle (CPA) tumor surgery, an analysis of cases was done.
Methods: In 37 patients who underwent CPA surgery, FNMEPs through corkscrew electrodes positioned at C5-C6 and C6-C5 (C is the central line of the brain as per 10–20 EEG electrode placement) were used to deliver short train stimuli and recorded from the orbicularis oculi, oris, and mentalis muscles.
Results: In 58 patients, triggered electromyography (EMG) was able to identify the FN during resection of tumor, but 8 out of these (4.64%) patients developed new facial weakness, whereas 3 out of 38 (1.11%) patients who had intact FN function MEP (decrement of FN target muscles – CMAPs amplitude peak to peak >50–60%), developed new facial weakness (House and Brackmann grade II to III).
Conclusion: The FNMEP has significant superiority over triggered EMG when tumor is giant and envelops the FN.
Keywords: Cerebellopontine angle tumors (CPA), Facial nerve motor evoked potential (MEP), Facial nerve preservation, Intraoperative neuromonitoring
INTRODUCTION
Skull base micro neurosurgery, especially of the cerebellopontine angle (CPA), puts the cranial nerves at risk, especially the facial nerve (FN). Among the technical measures to preserve FN function, intraoperative neuromonitoring (IONM) has become mandatory and is constantly being advanced.[
In this study, we present our experience in the use of FNMEP in predicting FN functional integrity following CPA surgery.
MATERIALS AND METHODS
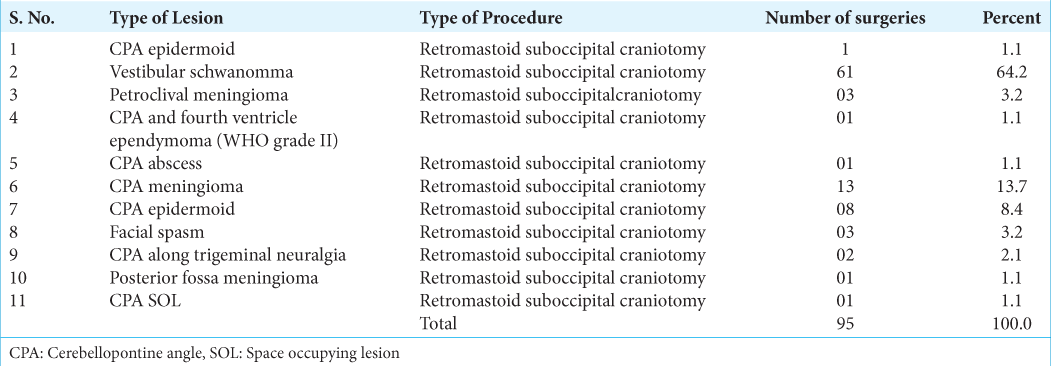
In this retrospective single-center study, we included all age patients who underwent IONM-guided CPA tumor surgery from April 2014 to December 2020 at the Sakra World Hospital, Bengaluru, India. All patients underwent monitoring with triggered electromyogram and spontaneous electromyogram for FN, out of which 37 underwent corticobulbar tract transcranial electrical MEP (CBT and TCeMEP), CBT, TCeMEP were recorded from upper and lower facial muscles. Standard demographic and clinical data were collected from all the enrolled patients. The preoperative House-Brackmann Grading scale was used to grade facial weakness. Retromastoid suboccipital craniotomy was done for all patients.
Total intravenous anesthesia was given. Neuromuscular block agents were used for intubation and omitted after that. Inhalational agent and neuromuscular blockade were avoided after intubation.
IONM was conducted using a Neuro Monitoring System (Medtronic Xomed, Jacksonville, FL, USA) NIM ECLIPSE E4 Version. CBT and TCeMEP were recorded using corkscrews electrode (DME1001 24K 1.2M CORKSCR ROHS Medtronic), which were placed according to 10–20 system interhemispheric CBT and TCeMEP montage C5-Anode-C6-Cathode, C6-Cathode-C5-Anode. This new term we used to define the location of the stimulating electrode. This is 10% of T3-C3, T4-C4 of 10–20 electro encephalography (EEG) electrode placement. To elicit the responses, we used a short train 2–3 pulses interstimulus interval ISI 1.2 ms, 75 µs pulse duration. The least current required of 90–110 Volt after wearing off of muscle relaxant to avoid the volume conduction from surrounding and other motor pathways. MEP responses were recorded from orbicularis oculi, orbicularis oris, and mentalis at the surgical site. A minimum amplitude of >50 µV with appropriate response latency was considered a reliable MEP response through twisted subdermal twisted-pair non-insulated straight needle electrodes (DSN2280 12K 27G 2.0M DL ROHS Medtronic). The recording conditions included a band-pass filter at 30–1500 Hz, with a 50 Hz Notch filter, and a Sweep speed of 5 ms/division window of 50 ms with autosensitivity.
RESULTS
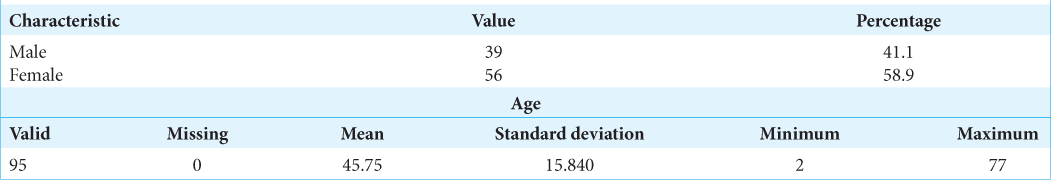
The study conducted included a total of 95 patients, comprising 39 men and 56 women, with an average age of 45.75 years. These patients underwent surgery for CPA tumors between April 2014 and December 2020. The age range of the patients was quite diverse, spanning from 2 months to 77 years, reflecting the broad spectrum of individuals affected by such tumors.
During the surgical procedures, different monitoring techniques were employed to ensure the safety and integrity of the FN. In 37 patients, transcranial FNMEP (TcFNMEP) was utilized, whereas triggered EMG and other monitoring modalities were utilized in the remaining cases.
Among the 58 patients who had intact FN function, trigger EMG was performed on all individuals to identify the FN during tumor resection. However, it was noted that 8 out of these 58 patients (4.64%) developed new facial weakness postoperatively. Similarly, in the subset of 37 patients who underwent TcFNMEP monitoring, 3 patients (1.11%) experienced a decrement in FN target muscle compound muscle action potentials (CMAPs) amplitude peak to peak of more than 50–60%. Notably, this observation occurred despite using the same current range (110–150 V) during the intraoperative period.
Following the surgeries, all patients who experienced new facial weakness, regardless of the monitoring technique used, presented with weakness graded between II and III. However, encouragingly, all of these patients eventually recovered to their preoperative status within 6–8 months.
The demographic characteristics of the patients included in the study are detailed in
Overall, the study underscores the significance of intraoperative monitoring techniques in safeguarding critical neural structures, such as the FN, during complex surgical procedures, ultimately contributing to improved patient outcomes and recovery.
Statistical analysis
DISCUSSION
Merton and Morton first reported an FNMEP elicited by transcranial electrical stimulation in 1980[
This monitoring approach records the CMAP in the target muscle (orbicularis oculi, orbicularis oris, and mentalis) evoked by stimulating the FN motor cortex.[
The alarm criteria of FNMEP have yet to be established. Some scholars recommend a >50% reduction in amplitude as an indicator for predicting FN damage [
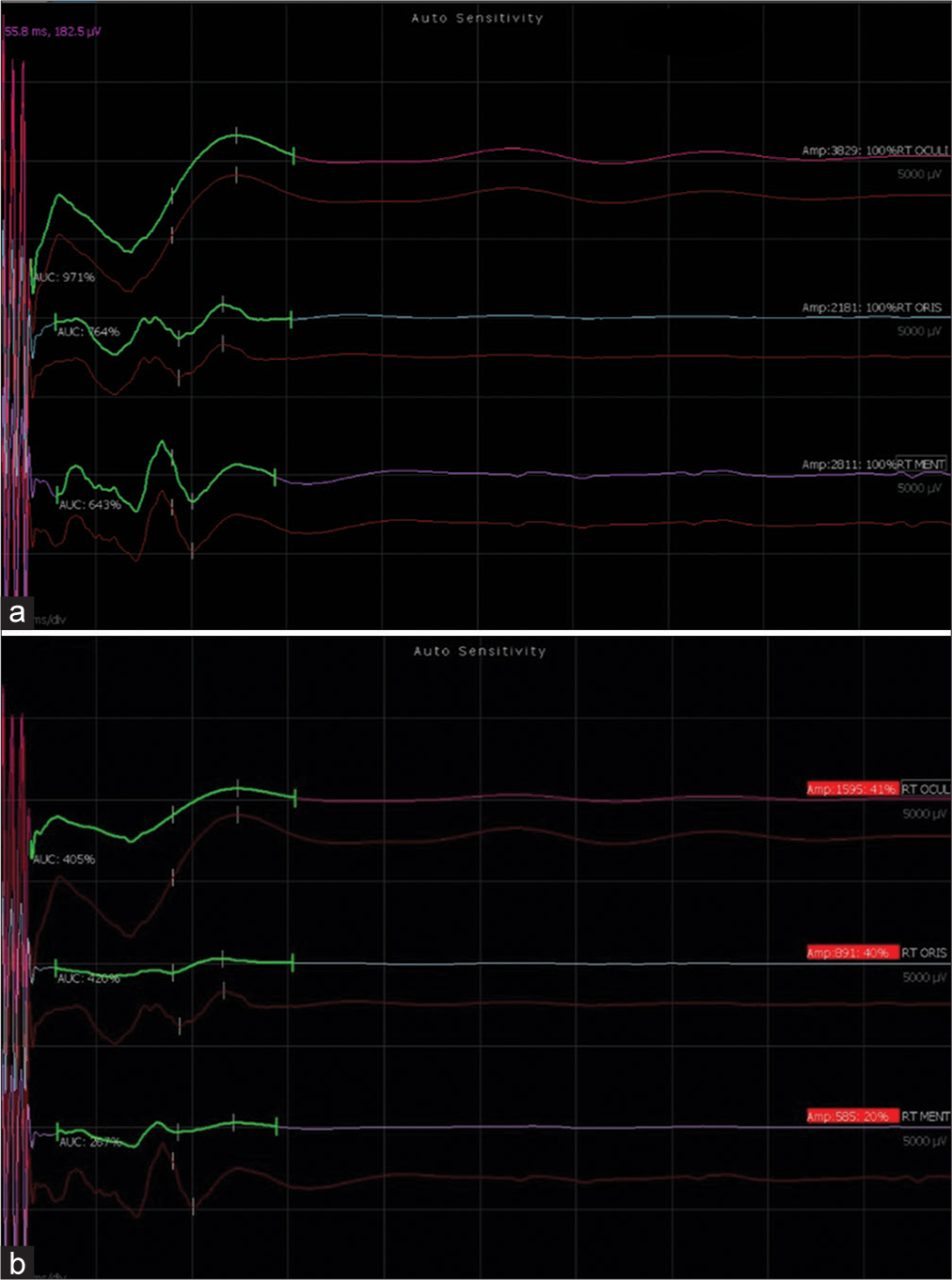
Figure 1:
During the case of acoustic schwannoma amplitude of corticobulbar tract, transcranial electrical motor evoked potential dropped while resecting the tumor at cerebellopontine angle: (a) a baseline trace, (b) showed dropped cranial nerves compound muscle action potentials (CMAPs) amplitude >50%.
In general, FN dysfunction immediately after surgery gradually resolves over the long term if the FN is anatomically preserved intraoperatively.[
Electromyographic activity occurs only at the time of direct irritation of the FN, while the absence of EMG activity may indicate a structurally and functionally intact nerve or a total loss of nerve function.[
EMG monitoring and triggered CMAP are not always satisfactory and not always predictive of FN function.[
In our center, we have used TcFNMEP as a complementary modality to direct nerve stimulation (triggered EMG) in our endeavor to preserve the FN during CPA tumor resection. Triggered EMG helps more in localizing the FN and predicting immediate nerve function, while FNMEP helps in monitoring the anatomical continuity of the nerve and also predicts long-term FN function.
In three of our patients, an intra-operative drop of more than 50% from baseline FNMEP was observed. Each of them developed House-Brackmann grade I-II facial weakness in the immediate postoperative period. However, at 6-month follow-up, all of them regained their preoperative FN functional status.
CONCLUSION
FN MEP is an emerging technique in the field of intra-operative neuromonitoring for CPA tumor surgery. When used judiciously in conjunction with triggered EMG, it can play a pivotal role in intra-operatively localizing the FN and predicting long-term FN function.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Acioly MA, Liebsch M, de Aguiar PH, Tatagiba T. Facial nerve monitoring during cerebellopontine angle and skull base tumor surgery: A systematic review from description to current success on function prediction. World Neurosurg. 2013. 80: e271-300
2. Akagami R, Dong CC, Westerberg BD. Localized transcranial electrical motor evoked potentials for monitoring cranial nerves in cranial base surgery. Neurosurgery. 2005. 57: 78-85
3. Arriaga MA, Luxford WM, Atkins JS, Kwartler JA. Predicting long-term facial nerve outcome after acoustic neuroma surgery. Otolaryngol Head Neck Surg. 1993. 108: 220-4
4. Bhimrao SK, Le TN, Dong CC, Makarenko S, Wongprasartsuk S, Westerberg BD. Role of facial nerve motor-evoked potential ratio in predicting facial nerve function in vestibular schwannoma surgery both immediate and at 1 year. Otol Neurotol. 2016. 37: 1162-7
5. Calancie B, Molano MR. Alarm criteria for motor-evoked potentials: What’s wrong with the “presence-or-absence” approach?. Spine (Phila Pa 1976). 2008. 33: 406-14
6. Deletis V, Fernandez-Conejero I. Intraoperative monitoring and mapping of the functional integrity of the brainstem. J Clin Neurol. 2016. 12: 262-73
7. Dong CC, Macdonald DB, Akagami R, Westerberg B, Alkhani A, Kanaan I. Intraoperative facial motor evoked potential monitoring with transcranial electrical stimulation during skull base surgery. Clin Neurophysiol. 2005. 116: 588-96
8. Fukuda M, Oishi M, Hiraishi T, Saito A, Fujii Y. Intraoperative facial nerve motor evoked potential monitoring during skull base surgery predicts long-term facial nerve function outcomes. Neurol Res. 2011. 33: 578-82
9. Goldbrunner RH, Schlake HP, Milewski C, Tonn JC, Helm J, Roosen K. Quantitative parameters of intraoperative electromyography predict facial nerve outcomes for vestibular schwannoma surgery. Neurosurgery. 2000. 46: 1140-8
10. Goto T, Muraoka H, Kodama K, Hara Y, Yako T, Hongo K. Intraoperative monitoring of motor evoked potential for the facial nerve using a cranial peg-screw electrode and a “threshold-level” stimulation method. Skull Base. 2010. 20: 429-34
11. Holland NR. Intraoperative electromyography. J Clin Neurophysiol. 2002. 19: 444-53
12. Liu SW, Jiang W, Zhang HQ, Li XP, Wan XY, Emmanuel B. Intraoperative neuromonitoring for removal of large vestibular schwannoma: Facial nerve outcome and predictive factors. Clin Neurol Neurosurg. 2015. 133: 83-9
13. Merton PA, Morton HB. Stimulation of the cerebral cortex in the intact human subject. Nature. 1980. 285: 227
14. Moller AR, Jannetta PJ. Preservation of facial function during removal of acoustic neuromas: Use of monopolar constant-voltage stimulation and EMG. J Neurosurg. 1984. 61: 757-60
15. Nanda A, Chittiboina P. Facial nerve monitoring in posterior fossa surgery. World Neurosurg. 2013. 80: e197-8
16. Oh T, Nagasawa DT, Fong BM, Trang A, Gopen Q, Parsa AT. Intraoperative neuromonitoring techniques in the surgical management of acoustic neuromas. Neurosurg Focus. 2012. 33: E6
17. Rampp S, Illert J, Krempler K, Strauss C, Prell J. A-train clusters and the intermedius nerve in vestibular schwannoma patients. Clin Neurophysiol. 2019. 130: 722-6
18. Romstöck J, Strauss C, Fahlbusch R. Continuous electromyography monitoring of motor cranial nerves during cerebellopontine angle surgery. J Neurosurg. 2000. 93: 586-93
19. Samii M, Gerganov V, Samii A. Improved preservation of hearing and facial nerve function in vestibular schwannoma surgery via the retrosigmoid approach in a series of 200 patients. J Neurosurg. 2006. 105: 527-35
20. Sarnthein J, Hejrati N, Neidert MC, Huber AM, Krayenbühl N. Facial nerve motor evoked potentials during skull base surgery to monitor facial nerve function using the threshold-level method. Neurosurg Focus. 2013. 34: E7
21. Sobottka SB, Schackert G, May SA, Wiegleb M, Reiss G. Intraoperative facial nerve monitoring (IFNM) predicts facial nerve outcome after resection of vestibular schwannoma. Acta Neurochir (Wien). 1998. 140: 235-43
22. Tsutsui S, Yamada H. Basic principles and recent trends of transcranial motor evoked potentials in intraoperative neurophysiologic monitoring. Neurol Med Chir. 2016. 56: 451-6
23. Vivas EX, Carlson ML, Neff BA, Shepard NT, McCracken DJ, Sweeney AD. Congress of neurological surgeons systematic review and evidence-based guidelines on intraoperative cranial nerve monitoring in vestibular schwannoma surgery. Neurosurgery. 2018. 82: E44-6.K
24. Zhou HH, Kelly PJ. Transcranial electrical motor evoked potential monitoring for brain tumor resection. Neurosurgery. 2001. 48: 1075-81