- Department of Neurosurgery, Toho University Graduate School of Medicine, Meguroku, Japan
- Department of Neurosurgery, Seisho Hospital, Odawara, Japan
- Department of Neurosurgery, Toho University Ohashi Medical Center, Meguroku, Japan
Correspondence Address:
Kazuma Tsuto, Department of Neurosurgery, Toho University Graduate School of Medicine, Meguroku, Japan.
DOI:10.25259/SNI_709_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kazuma Tsuto1, Masataka Takeuchi2, Yu Shimizu2, Takashi Matsumoto2, Satoshi Iwabuchi3. Frictional forces in stent retriever procedures: The impact of vessel diameter, angulation, and deployment position. 25-Oct-2024;15:384
How to cite this URL: Kazuma Tsuto1, Masataka Takeuchi2, Yu Shimizu2, Takashi Matsumoto2, Satoshi Iwabuchi3. Frictional forces in stent retriever procedures: The impact of vessel diameter, angulation, and deployment position. 25-Oct-2024;15:384. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13163
Abstract
Background:Mechanical thrombectomy has improved the outcome of patients with acute ischemic stroke, but complications such as subarachnoid hemorrhage (SAH) can worsen the prognosis. This study investigates the frictional forces exerted by stent retrievers (SRs) on vessel walls, hypothesizing that these forces contribute to vascular stress and a risk of hemorrhage. We aimed to understand how vessel diameter, curvature, and stent deployment position influence these forces.
Methods:Using a silicone vascular model simulating the middle cerebral artery, we created virtual vessels with diameters of 2.0 mm and 2.5 mm, each with branching angles of 60° and 120°. A Trevo NXT (4 × 28 mm) SR was deployed and retracted through these models, measuring the maximum static frictional force at the moment the SR began to move. The stent deployment position relative to the curvature (straight, distal 1/4, center, and proximal 1/4) was also varied to assess its impact on frictional forces. Each condition was tested 15 times, and the results were statistically analyzed.
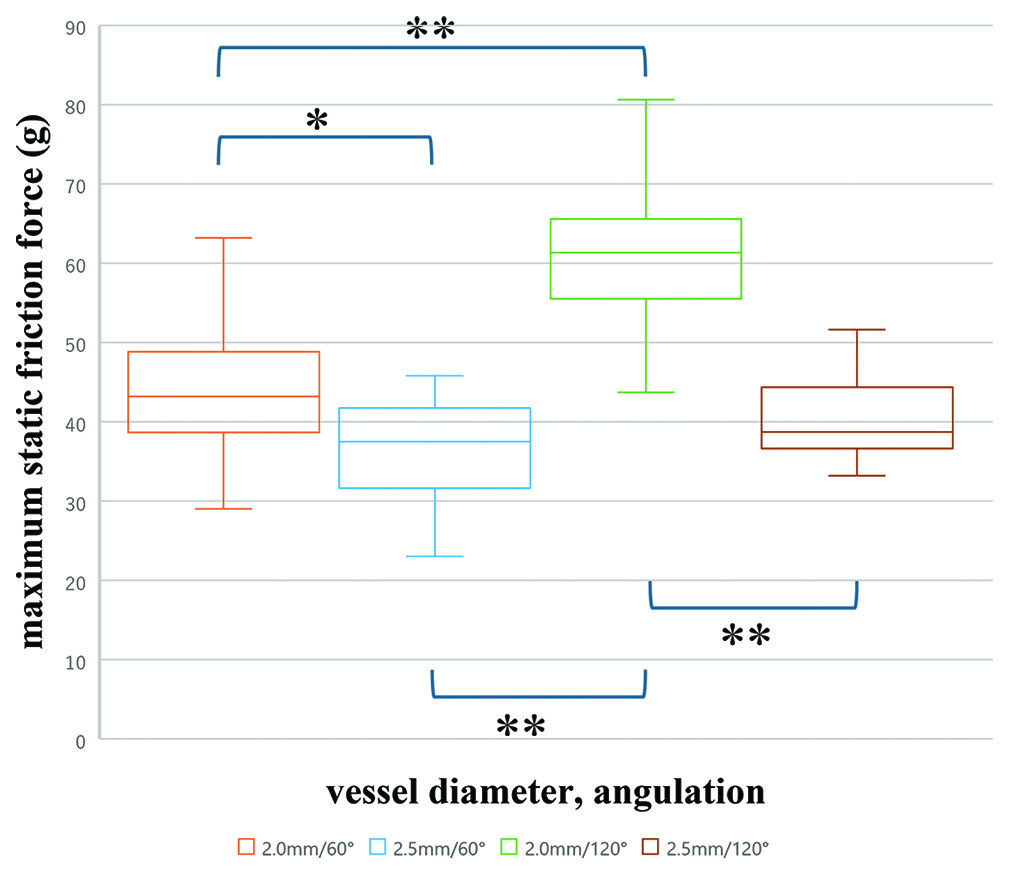
Results:The highest frictional force was observed in the 2.0 mm/120° model, followed by the 2.0 mm/60°, 2.5 mm/120°, and 2.5 mm/60° models. Narrower and more sharply curved vessels exhibited significantly higher frictional forces. Friction also increased with more distal stent deployment, particularly in the narrower vessels.
Conclusion:Smaller vessel diameters, greater curvature, and more distal stent deployment positions increase frictional forces during thrombectomy, potentially leading to SAH. These findings highlight the importance of selecting appropriately sized SRs and considering stent deployment positions to minimize vascular stress.
Keywords: Frictional forces, Mechanical thrombectomy, Stent retrievers, Vascular curvature, Vessel diameter
INTRODUCTION
In recent years, remarkable progress in mechanical thrombectomy for acute cerebral vessel occlusion has led to improved outcomes in ischemic stroke treatment. However, hemorrhagic complications during thrombectomy can adversely affect patient prognosis.[
MATERIALS AND METHODS
A cylindrical cavity was created in a plate-shaped silicone material to simulate virtual blood vessels, specifically modeling the middle cerebral artery’s (MCA) M1 and M2 segments based on several clinical studies.[
Figure 2:
An experimental model for measuring stent retrieval frictional force. (a) Overview of the model. (b) The stent retriever is deployed within the vascular model filled with 37°C surfactant. (c) To ensure consistent stent deployment positions, the vascular model was captured on camera, and the position of the stent’s distal end was marked on an enlarged monitor. (d) The delivery wire of the stent was passed through the guiding catheter. (e) The proximal end of the delivery wire was fixed to a gripper attached to a digital indicator. The yellow arrows indicate the action of traction applied by the traction device.
A traction device was attached to the delivery wire of the deployed SR, and linear traction was applied at a speed of 1 cm per second [
Statistical analysis
Statistical differences were analyzed using Bartlett’s test to assess the homogeneity of variances across groups. If the variances were equal, a one-way analysis of variance (ANOVA) was conducted; if they were unequal, the Kruskal–Wallis test was used. Multiple comparisons were performed using Tukey’s post hoc test following ANOVA and the Steel–Dwass method following the Kruskal–Wallis test. A P < 0.05 was considered statistically significant. All statistical analyses were conducted using Statcel, an Excel add-in (4th edition).
RESULTS
In all procedures, peak load on the traction device was observed at the moment the SR began to move.
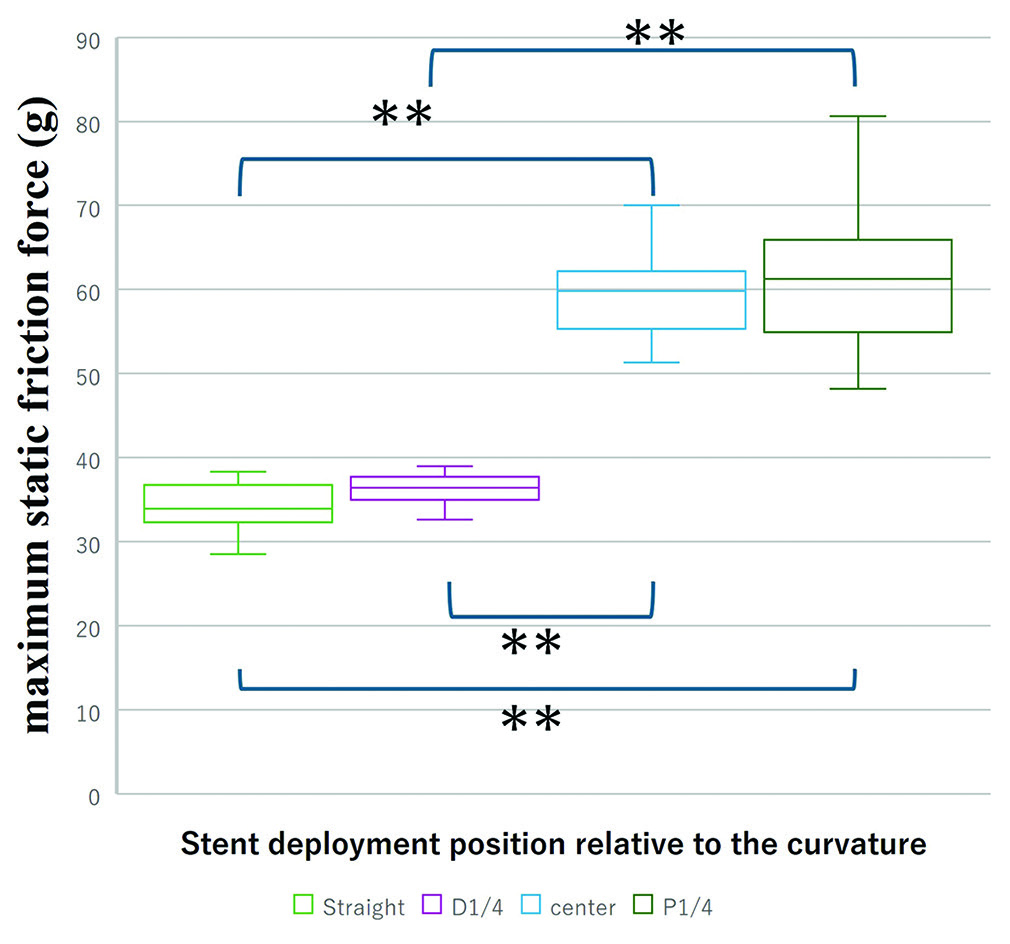
The maximum static friction force exerted when the SR began to move was highest in the vessel with a 2.0 mm diameter and a 120° curvature (mean ± standard deviation: 60.7 ± 9.0 g), followed by the 2.0 mm/60° (44.4 ± 9.1 g), the 2.5 mm/120° (40.4 ± 6.0 g), and the 2.5 mm/60° (36.8 ± 6.6 g) conditions, in that order. Significant differences were observed among these four groups (one-way ANOVA; P < 0.01). Pairwise comparisons revealed that in vessels with a smaller diameter (2.0 mm), a greater curvature resulted in a significantly higher friction force. Conversely, in vessels with a larger diameter (2.5 mm), increasing the curvature did not lead to significant differences in friction force (Tukey–Kramer method). Furthermore, the maximum static friction force in the straight section of the 2.0 mm lumen model was 34.2 ± 3.0 g. Significant differences were observed among the four conditions where the stent deployment position varied relative to the curved section, with a tendency for stronger traction forces as the deployment position moved more distally (Kruskal–Wallis test) [
Figure 4:
Comparison of maximum static friction force among different stent deployment positions relative to the curvature. The box plots illustrate the frictional forces measured when the stent is deployed in various positions: straight, distal 1/4 (D1/4), center, and proximal 1/4 (P1/4) relative to the curvature. In the Kruskal–Wallis test with the Steel–Dwass method, **P < 0.01.
However, in comparisons among the groups, no significant differences in traction forces were found between the group where the stent was deployed in the straight section and the group where the distal 1/4 of the stent was positioned in the curved area (36.4 ± 2.6 g). Similarly, no significant differences were found between the group where the central portion of the stent was aligned with the curved section (59.4 ± 4.9 g) and the group where the proximal 1/4 was positioned in the same area (Steel–Dwass method).
DISCUSSION
When thrombectomy using SRs is performed for occlusions in the distal MCA, the recanalization rate is generally high. However, SAH is reported to occur more frequently compared to occlusions in the proximal MCA or internal carotid artery (ICA).[
In our experiment, we hypothesized that the maximum static friction force applied to the traction device at the moment the SR starts to move corresponds to the force that causes vascular displacement.
This study is the first to investigate how vascular diameter and curvature affect frictional forces between an SR and the vessel using a vascular model. Machi et al., similarly demonstrated that greater friction occurs when withdrawing an SR within a narrower tube[
Another concern when using an SR in cerebral blood vessels is the potential for vascular damage during the retrieval process. Shibata et al. reported a case of vessel perforation during thrombectomy with a 6-mm SR in a single pass for tandem occlusion of the ICA and distal M1,[
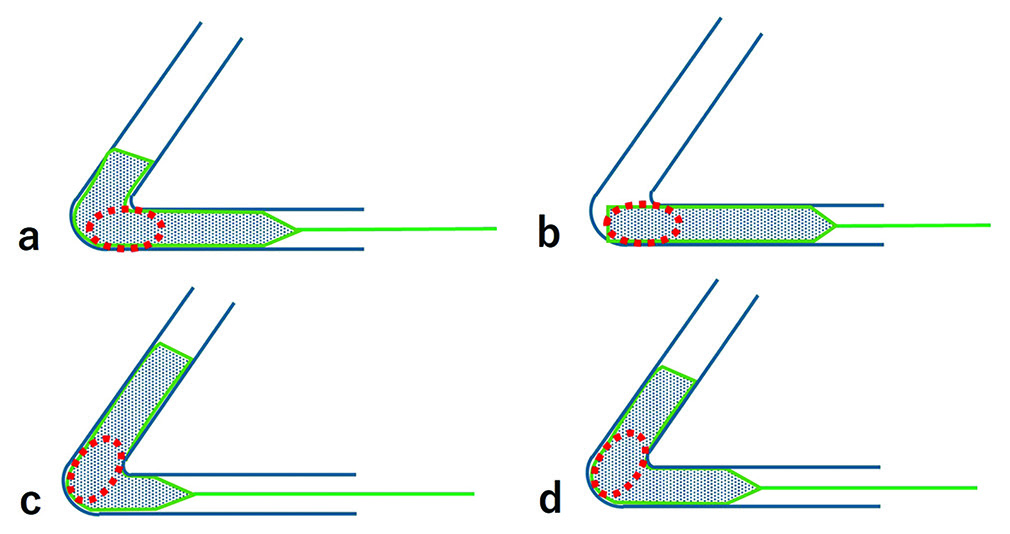
In the SR procedure, it is important to minimize the burden on the vessel, as well as to maximize the probability of successful reperfusion. Achieving both goals may require careful consideration of the stent’s deployment position relative to the vessel’s curvature. To enhance thrombus capture by the stent struts, the stent should be deployed more distally relative to the thrombus. However, Funatsu et al. reported that when the SR was positioned more distally, it often encountered vascular wall components within the thrombus,[
Figure 5:
Illustration showing the differences in stent deployment positions relative to the curved section of a blood vessel. (a) Stent deployed in the straight section, (b-d) Stent deployed with different sections (distal 1/4, center, proximal 1/4) aligned with the curve. The red circles indicate the location of the thrombus.
To better understand how SRs function in the human cerebral artery, a model that closely resembles the actual vascular environment is required. Kwak et al. developed a cerebral artery model using 3D printing based on real human cerebral angiograms.[
Our study has certain limitations. First, it is an in vitro study using a rigid silicone model, which means that the vessels did not deform as they would in real-life conditions. In actual thrombectomy procedures within human cerebral arteries, vessels are expected to deform during SR retrieval, and our study could not assess the extent to which this deformation contributes to vessel stress. The accordion-like shortening along the longitudinal axis, often observed in real cerebral vessels, is difficult to replicate. Second, as previously mentioned, various factors such as vessel elasticity, endothelial characteristics, blood flow, number of bends, and properties and length of the thrombus play a role in the clinical environment.[
CONCLUSION
Our study findings show that frictional forces exerted by SRs on vessel walls are significantly influenced by vessel diameter, curvature, and stent deployment position. Smaller diameters, greater curvature, and more distal stent deployment result in higher frictional forces during thrombectomy, potentially increasing to the risk of hemorrhagic complications such as SAH. These findings highlight the necessity of selecting SRs appropriately sized for the vessel and carefully considering deployment position to minimize vascular stress. Future studies with models that better replicate human cerebral arteries are essential to further validate these results and optimize thrombectomy techniques.
Ethical approval
Institutional Review Board approval is not required as this study did not involve human subjects and presented results from experimental models; therefore, we determined that ethical approval was not required, and this was confirmed by the ethics committee of our institution.
Declaration of patient consent
Patient’s consent is not required as there are no patients in this study.
Financial support and sponsorship
The study was financially supported by Kaneka Medix.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Baharvahdat H, Ooi YC, Khatibi K, Ponce Mejia LL, Kaneko N, Nour M. Increased rate of successful first passage recanalization during mechanical thrombectomy for M2 occlusion. World Neurosurg. 2020. 139: e792-e9
2. Charbonnier G, Bonnet L, Biondi A, Moulin A. Intracranial bleeding after reperfusion therapy in acute ischemic stroke. Front Neurol. 2021. 11: 629920
3. Funatsu N, Hayakawa M, Hashimoto T, Yamagami H, Satow T, Takahashi JC. Vascular wall components in thrombi obtained by acute stroke thrombectomy: Clinical significance and related factors. J Neurointerv Surg. 2019. 11: 232-6
4. Gunning GM, MacArdle K, Mirza M, Duffy S, Gilvarry M, Brouwer PA. Clot friction variation with fibrin content; implications for resistance to thrombectomy. J Neurointerv Surg. 2018. 10: 34-8
5. Harker P, Aziz YN, Vranic J, Chulluncuy-Rivas R, Previtera M, Yaghi S. Asymptomatic intracerebral hemorrhage following endovascular stroke therapy is not benign: A systematic review and meta-analysis. J Am Heart Assoc. 2024. 13: e031749
6. Hao Y, Yang D, Wang H, Zi W, Zhang M, Geng Y. Predictors for symptomatic intracranial hemorrhage after endovascular treatment of acute ischemic stroke. Stroke. 2017. 48: 1203-9
7. He J, Fu F, Zhang W, Zhan Z, Cheng Z. Prognostic significance of the clinical and radiological haemorrhagic transformation subtypes in acute ischaemic stroke: A systematic review and meta-analysis. J Neurol. 2022. 29: 3449-59
8. Jiang F, Zhao W, Wu C, Zhang Z, Li C, Che R. Asymptomatic intracerebral hemorrhage may worsen clinical outcomes in acute ischemic stroke patients undergoing thrombectomy. J Stroke Cerebrovasc Dis. 2019. 28: 1752-8
9. Jumma MA, Castonguay AC, Salahuddin H, Jadhav AP, Limaye K, Farooqui M. Middle cerebral artery M2 thrombectomy in the STRATIS Registry. Stroke. 2021. 52: 3490-6
10. Kim DY, Baik SH, Jung C, Kim JY, Han SG, Kim BJ. Predictors and impact of sulcal SAH after mechanical thrombectomy in patients with isolated M2 occlusion. Am J Neuroradiol. 2022. 43: 1292-8
11. Kinjo N, Yoshimura S, Uchida K, Sakai N, Yamagami H, Morimoto T. Incidence and prognostic impact of intracranial hemorrhage after endovascular treatment for acute large vessel occlusion. Cerebrovasc Dis. 2020. 49: 540-9
12. Kniep H, Meyer L, Broocks G, Bechstein M, Guerreiro H, Winkelmeier L. Predictors of functional outcome after thrombectomy for M2 occlusions: A large scale experience from clinical practice. Sci Rep. 2023. 13: 18740
13. Kwak Y, Son W, Kim BJ, Kim M, Yoon SY, Park J. Frictional force analysis of stent retriever devices using a realistic vascular model: Pilot study. Front Neurol. 2022. 13: 964354
14. Lee H, Qureshi AM, Mueller-Kronast NH, Zaidat OO, Froehler MT, Liebeskind DS. Subarachnoid hemorrhage in mechanical thrombectomy for acute ischemic stroke: Analysis of the STRATIS registry, systematic review and meta-analysis. Front Neurol. 2021. 12: 663058
15. Liu D, Zhang G, Wang Y, Li J, Cao P, Yin X. Geometric features of middle cerebral artery are associated with spontaneous basal ganglia intracerebral haemorrhage. Stroke Vasc Neurol. 2022. 7: 399-405
16. Machi P, Jourdan F, Ambard D, Reynaud C, Lobotesis K, Sanchez M. Experimental evaluation of stent retrievers’ mechanical properties and effectiveness. J Neurointerv Surg. 2017. 9: 257-63
17. Mokin M, Fargen KM, Primiani CT, Ren Z, Dumont TM, Brasiliense LB. Vessel perforation during stent retriever thrombectomy for acute ischemic stroke: Technical details and clinical outcomes. J Neurointerv Surg. 2017. 9: 922-8
18. Mouches P, Forkert ND. A statistical atlas of cerebral arteries generated using multi-center MRA datasets from healthy subjects. Sci Data. 2019. 6: 29
19. Naggara O, Raymond J, Domingo M, Al-Shareef F, Touzé E, Chenoufi M. T2* “susceptibility vessel sign” demonstrates clot location and length in acute ischemic stroke. PLoS One. 2013. 8: e76727
20. Ng PP, Larson TC, Nichols CW, Murray MM, Salzman KL, Smith RH. Intraprocedural predictors of post-stent retriever thrombectomy subarachnoid hemorrhage in middle cerebral artery stroke. J Neurointerv Surg. 2019. 11: 127-32
21. Nikoubashman O, Reich A, Pjontek R, Jungbluth M, Wiesmann M. Postinterventional subarachnoid haemorrhage after endovascular stroke treatment with stent retrievers. Neuroradiology. 2014. 56: 1087-96
22. Nome T, Enriquez B, Nome CG, Tennøe B, Lund CG, Skjelland M. Clinical outcome after thrombectomy in patients with MeVO stroke: Importance of clinical and technical factors. J Neurointerv Surg. 2024. 271: 877-86
23. Renieri L, Valente I, Dmytriw AA, Puri AS, Singh J, Nappini S. Mechanical thrombectomy beyond the circle of Willis: Efficacy and safety of different techniques for M2 occlusions. J Neurointerv Surg. 2022. 14: 546-50
24. Shibata A, Yanagawa T, Sugasawa S, Ikeda S, Ikeda T. Multiple large vessel occlusions resulting in vessel perforation in single pass of mechanical thrombectomy with stent retriever. Radiol Case Rep. 2023. 18: 3206-11
25. Suzuki K, Katano T, Numao S, Nishi Y, Kutsuna A, Kanamaru T. The effect of asymptomatic intracranial hemorrhage after mechanical thrombectomy on clinical outcome. J Neurol Sci. 2024. 457: 122868
26. Tang G, Cao Z, Luo Y, Wu S, Sun X. Prognosis associated with asymptomatic intracranial hemorrhage after acute ischemic stroke: A systematic review and meta-analysis. J Neurol. 2022. 269: 3470-81
27. Teng D, Pannell JS, Rennert RC, Li J, Li YS, Wong VW. Endothelial trauma from mechanical thrombectomy in acute stroke: In vitro live-cell platform with animal validation. Stroke. 2015. 46: 1099-106
28. Yilmaz U, Walter S, Körner H, Papanagiotou P, Roth C, Simgen A. Peri-interventional subarachnoid hemorrhage during mechanical thrombectomy with stent retrievers in acute stroke: A retrospective case-control study. Clin Neuroradiol. 2015. 25: 173-6
29. Yoon W, Jung MY, Jung SH, Park MS, Kim JT, Kang HK. Subarachnoid hemorrhage in a multimodal approach heavily weighted toward mechanical thrombectomy with solitaire stent in acute stroke. Stroke. 2013. 44: 414-9