- Department ofAnesthesiology, All India Institute of Medical Sciences, Nagpur, Maharashtra, India,
- Department ofNeurosurgery, All India Institute of Medical Sciences, Nagpur, Maharashtra, India,
- Department of Neurosurgery, Nepalgunj Medical College, Nepalgunj, Nepal,
- Department of Neurosurgery, GB Pant Institute of Post Graduate Medical Education and Research, New Delhi, Delhi, India.
- Department of Anesthesiology, BLK Max Hospital, New Delhi, Delhi, India.
- Department of Neuroanesthesia, Max Saket Hospital, New Delhi, Delhi, India.
Correspondence Address:
Chayanika Kutum, Department of Anesthesiology, All India Institute of Medical Sciences, Nagpur, Maharashtra, India.
DOI:10.25259/SNI_412_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Chayanika Kutum1, Prashant Lakhe2, Niraj Ghimire3, Anil Kumar BC4, Uzma Begum5, Karandeep Singh6. Intraoperative goal-directed fluid therapy in neurosurgical patients: A systematic review. 05-Jul-2024;15:233
How to cite this URL: Chayanika Kutum1, Prashant Lakhe2, Niraj Ghimire3, Anil Kumar BC4, Uzma Begum5, Karandeep Singh6. Intraoperative goal-directed fluid therapy in neurosurgical patients: A systematic review. 05-Jul-2024;15:233. Available from: https://surgicalneurologyint.com/surgicalint-articles/12976/
Abstract
Background: Perioperative fluid management is critical in neurosurgery as over perfusion can lead to brain edema whereas under perfusion may lead to brain hypoperfusion or ischemia. We aimed to determine the effectiveness of intraoperative goal-directed fluid therapy (GDFT) in patients undergoing intracranial surgeries.
Methods: We searched MEDLINE, Cochrane, and PubMed databases and forward-backward citations for studies published between database inception and February 22, 2024. Randomized controlled trials where intraoperative GDFT was performed in neurosurgery and compared to the conventional regime were included in the study. GDFT was compared with the conventional regime as per primary outcomes – total intraoperative fluid requirement, serum lactate, hemodynamics, brain relaxation, urine output, serum biochemistry, and secondary outcomes – intensive care unit and hospital length of stay. The quality of evidence was assessed with the Cochrane risk of bias tool. This study is registered on PROSPERO (CRD42024518816).
Results: Of 75 records identified, eight were eligible, the majority of which had a low to moderate risk of overall bias. In four studies, more fluid was given in the control group. No difference in postoperative lactate values was noted in 50% of studies. In the remaining 50%, lactate was more in the control group. Three out of four studies did not find any significant difference in the incidence of intraoperative hypotension, and four out of six studies did not find a significant difference in vasopressor requirement. The majority of studies did not show significant differences in urine output, brain relaxation, and length of stay between both groups. None found any difference in acid base status or electrolyte levels.
Conclusion: GDFT, when compared to the conventional regime in neurosurgery, showed that the total volume of fluids administered was lesser in the GDFT group with no increase in serum lactate. There was no difference in the hemodynamics, urine output, brain relaxation, urine output, length of stay, and biochemical parameters.
Keywords: Fluid management, Goal-directed fluid therapy, Neuroanesthesia, Neurosurgery
INTRODUCTION
Optimal fluid administration during the intraoperative period is a vital component in the management of surgical patients. Hypovolemia, on the one hand, can lead to inadequate organ perfusion. Whereas, hypervolemia can cause interstitial edema, decreased tissue healing, local inflammation, increased wound infection, and wound dehiscence.[
MATERIALS AND METHODS
The current systematic review was conducted as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.[
Objective
The objective of this study was to determine the effectiveness of intraoperative GDFT in patients undergoing intracranial surgeries with regard to the following outcome variables:
Primary outcomes
Total intraoperative fluid requirement Serum lactate levels Intraoperative hemodynamics – Total number of hypotensive episodes, total vasopressor requirement Brain relaxation Urine output Serum biochemistry – pH, serum electrolytes
Secondary outcomes
Intensive care unit (ICU) and hospital length of stay.
Study selection criteria
Patient groups
Adult patients (over 18 years) undergoing elective or emergency craniotomy surgery were included in the study. The studies were not limited in terms of the type or location of the intracranial pathology.
Intervention and comparison
The patient participants had to be randomly assigned to either receive GDFT or conventional fluid management intraoperatively. We defined intraoperative GDFT as any fluid administration guided by continuously measured hemodynamic variables targeted to maximize tissue perfusion and oxygen delivery. These hemodynamic variables included cardiac output, stroke volume, SVV, PPV, or other factors, as measured by any device. Studies in which the control group also received any other form of GDFT were excluded from the study. Conventional fluid management was considered in the form of protocol-driven standard care, for example, maintaining mean arterial pressure > 65 mmHg or central venous pressure (CVP) >8 mmHg or care at the discretion of the attending physicians.
Types of studies
Randomized controlled trials (RCTs) where intraoperative GDFT was performed in adult patients scheduled for intracranial surgery. Non-randomized trials, cohort studies, retrospective studies, animal model trials, studies with incomplete text, and studies in languages other than English were excluded from the study.
Search strategies and data collection
The literature search was conducted on PubMed, MEDLINE, and Science Direct. Keywords for database search included the terms “goal-directed fluid neurosurgery.” The last search was done on February 22, 2024. Independent reviewers screened the articles for titles and abstracts. Studies were “included” if the selection criteria were met. In case of doubt, if any, they were resolved by the other author. Full-text articles were retrieved. The final inclusion of any study was based on full-text reading. Two review authors independently extracted data from the included studies, and a third review author rechecked the data. The reference lists were scanned, and any relevant citations were identified and included for analysis. A spreadsheet-based data extraction form was used to collect study information. We extracted the following study characteristics:
General information: Year of study Methods: Study design, randomization method, and blinding method Participants: Total number (n), age range, types of surgery, comorbidities, inclusion criteria, and exclusion criteria Interventions: Intervention, comparison, medications, or interventions excluded Outcomes: Primary and secondary outcomes specified and collected Notes: Funding for study and conflicts of interest of study authors.
As only qualitative analysis of available data was planned, alternative data synthesis methods and meta-analyses were not considered.
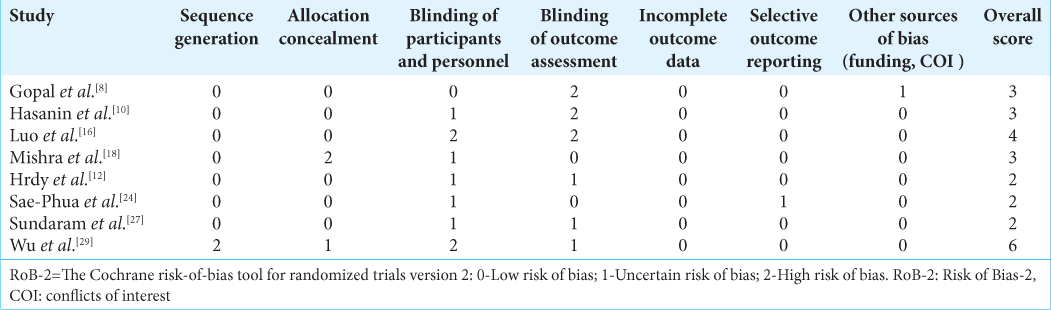
Risk of bias assessment
The risk of bias and the certainty assessment were done by risk of bias-2 by two review authors.[ Random sequence generation Allocation concealment Blinding of participants and personnel Blinding of outcome assessment Incomplete outcome data Selective outcome reporting Other potential bias
Studies were considered to be at low risk of bias if they adequately met the first five criteria with no evidence of significant selective reporting bias or any other major sources of bias.
RESULTS
Literature search and study selection
PubMED, Science Direct and Cochrane search initially retrieved 75 citations with 7 RCTs meeting the inclusion criteria [PRISMA Flowchart,
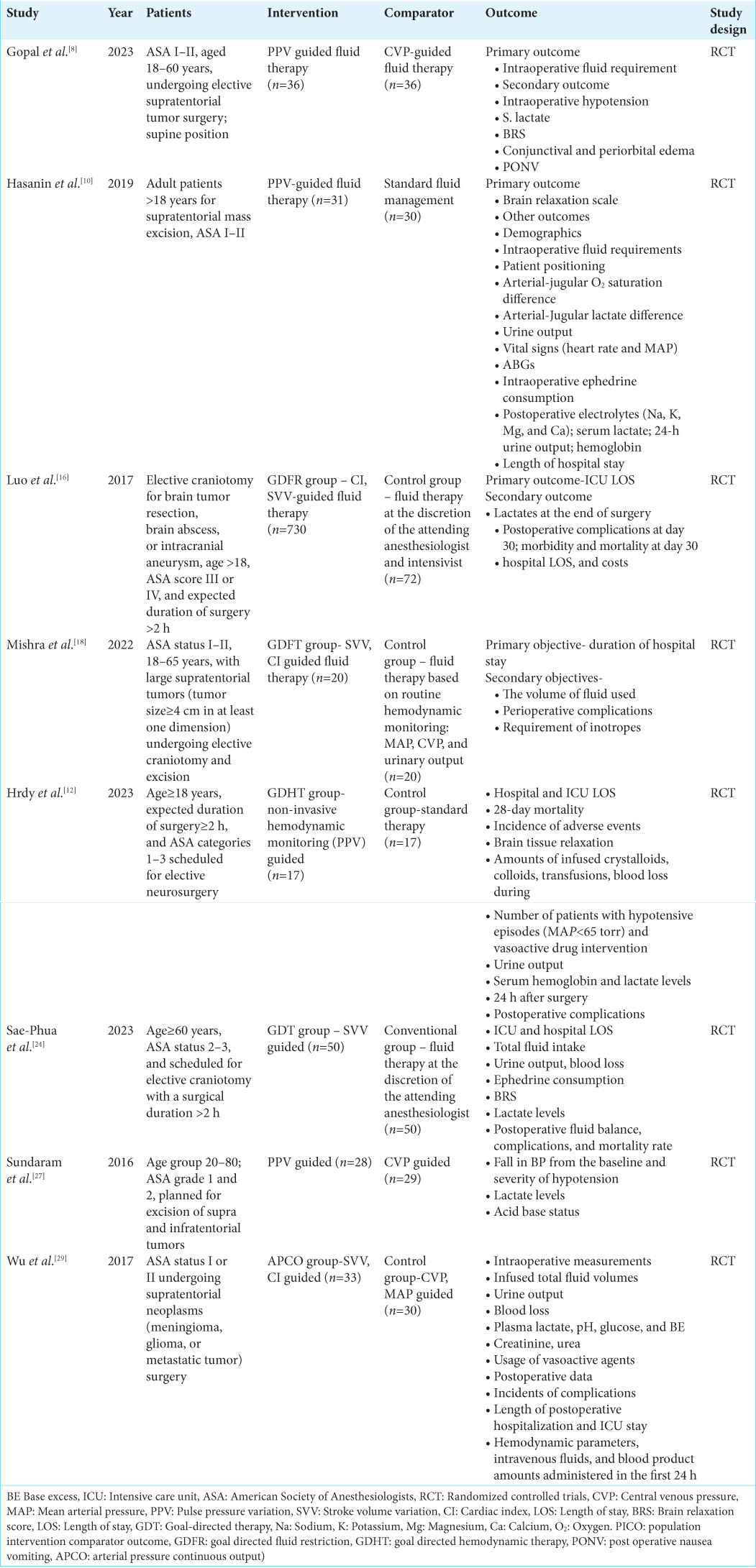
The studies included in this review were heterogeneous and varied in design [
Baseline characteristics of included RCTs
The salient baseline characteristics of included RCTs are shown in
As per the risk of bias assessment tool, the overall score was two for three studies,[
Three (37.5%) studies considered PPV as the therapeutic goal for GDFT.[
Primary outcomes
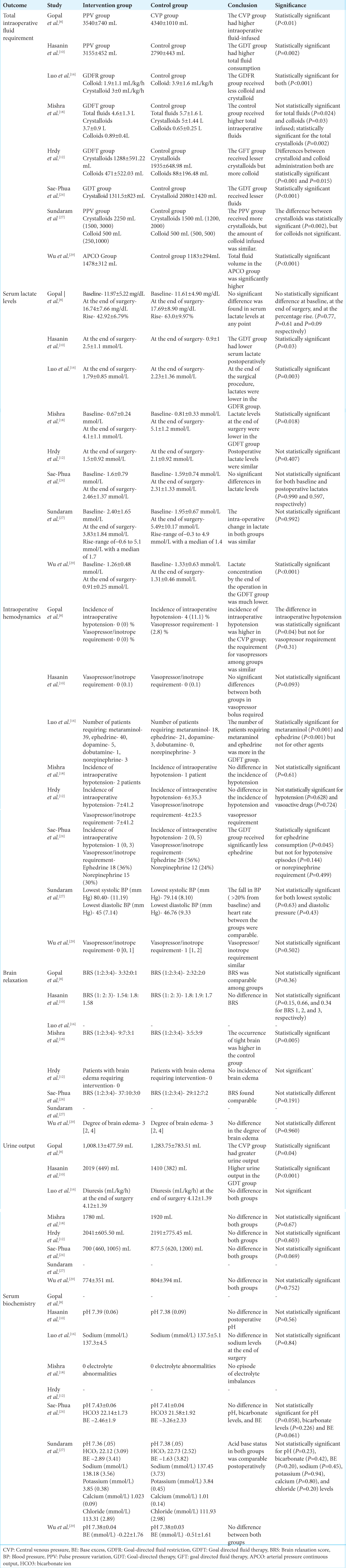
Total intraoperative fluid requirement
The total intraoperative fluid requirement was significantly higher in the control group in 3 (37.5%) studies [
Serum lactate levels
Serum lactate levels at the end of surgery were significantly higher in the control group than in the GDFT group in 4 (50%) studies [
Intraoperative hemodynamics
Four authors mentioned the incidence of intraoperative hypotension [
Brain relaxation
Six out of eight studies studied brain relaxation in both groups [
Urine output
Seven out of eight studies noted the urine output of patients [
Serum biochemistry
Six out of eight studies examined the serum biochemical parameters, that is, pH and electrolytes [
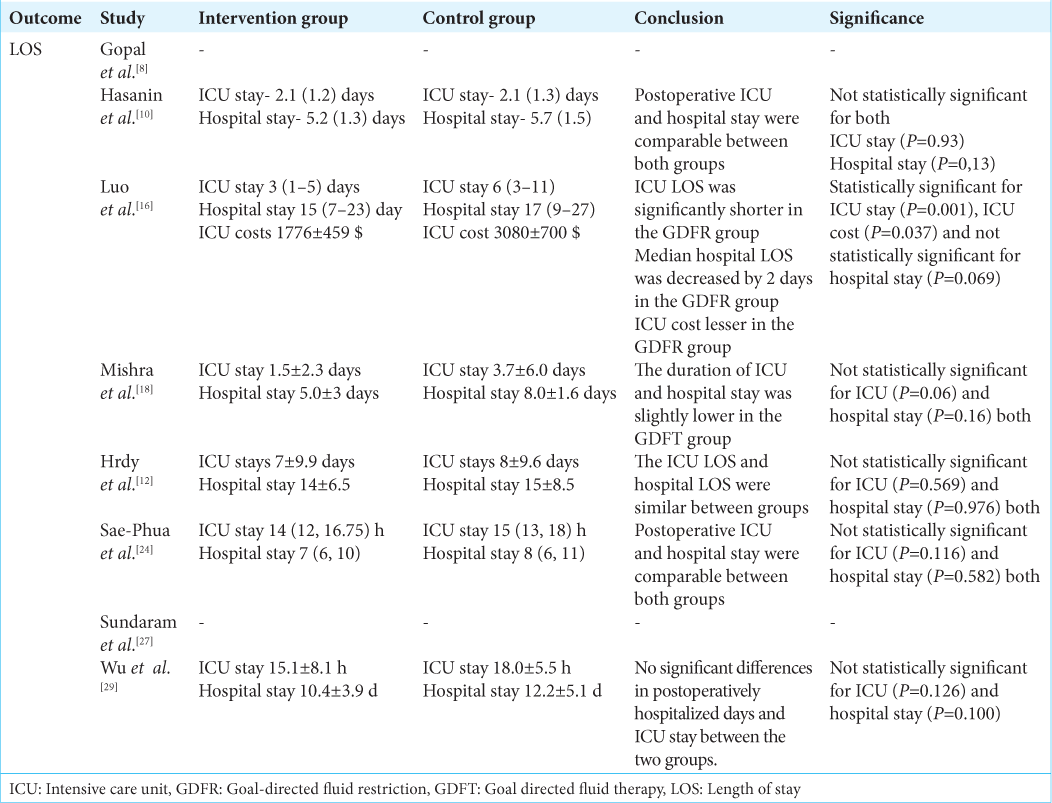
Secondary outcomes
ICU and hospital length of stay
Six out of eight studies examined the ICU and hospital length of stay of patients [
DISCUSSION
This systematic review included eight RCTs comparing GDFT with conventional regimes for intraoperative fluid administration in neurosurgery with a total of 572 patients.
It was found that in the majority of studies that compared the total intraoperative fluid administration, more fluid was given in the control group. The postoperative serum lactate values were similar between both groups in 50% of the studies. Whereas in the remaining half studies, it was found to be more in the control group. Regarding hemodynamics, the majority of studies did not find any significant difference in the incidence of intraoperative hypotension and vasopressor requirement in both groups. Similarly, in a greater number of studies, there was no significant difference in the urine output, brain relaxation, and length of stay between both groups. None of the studies found any difference in the acid-base status or electrolyte levels between the groups.
The main aim of perioperative fluid management is to maintain an optimum cardiac output and tissue perfusion. GDFT utilizes certain hemodynamic targets such as PPV, SVV, and CI to identify the fluid responsiveness of patients. This helps in preventing fluid overload in patients and the deleterious effects such as pneumonia, respiratory failure, pulmonary edema, and delayed wound healing.[
Perioperative fluid management is critical in neurosurgery as over perfusion can lead to brain edema, whereas under perfusion may lead to brain hypoperfusion or ischemia. Other concerning points specific to neurosurgery are the use of osmotic diuretics, the significance of the type of fluid used, the probability of long duration surgeries, major fluid shifts, difficult assessment of blood loss under the drapes, intraoperative diabetes insipidus, and distinct type of surgeries such as vascular surgeries which require unique fluid management. Thus, it becomes essential to use a proper parameter to guide fluid management in neurosurgery. To date, only one meta-analysis has been done, which individually evaluated the effect of GDFT on neurosurgical patients.[
As per the results, it is seen that the majority of the authors found that the intraoperative fluid administration was higher in the group following the conventional fluid management strategy.[
It was observed that the serum lactate levels were either similar or higher in the control group. It is noteworthy that in none of the studies the serum lactate was higher in the patients receiving GDFT. This point indicates that GDFT might be able to maintain adequate end organ perfusion. A retrospective cohort study demonstrated that elevated intraoperative serum lactate in craniotomy patients is associated with new neurological deficits and longer length of stay.[
In our review, we only included studies comparing GDFT versus conventional fluid management in neurosurgery. However, few other studies in the literature have compared two different methods of GDFT in neurosurgery. PPV or SVV constitute the dynamic variables for predicting fluid responsiveness with no fixed single cut off value. Both these parameters have a “gray zone” of two cutoffs within which the validity is inconclusive.[
Another RCT by Nayak et al. compared PPV with pleth variability index (PVI) in patients undergoing supratentorial lesion surgeries.[
PPV and SVV both serve as a reliable dynamic parameter to assess fluid responsiveness provided that the physiological limitations are avoided.[
Hrdy et al. utilized a non-invasive method of assessing stroke volume for GDFT.[
Luo et al. observed that in the GDFT group, the ICU expenses were significantly lower than in the conventional group.[
However, our systematic review had few limitations. The study population was heterogeneous. Sae-Phua et al. included only elderly patients aged more than 60 years, unlike the rest of the studies.[
The strength of our systematic review is that all the included studies were relatively new, that is, within the year 2016–2023. No other review has been done in the literature exclusively assessing GDFT in the neurosurgical patient population.
CONCLUSION
Perioperative optimal fluid management plays an important role in neurosurgical patients. GDFT, when compared to conventional regime in neurosurgery showed that the total volume of fluids administered was lesser in the GDFT group with no increase in serum lactate levels. However, there was no difference in the hemodynamics, urine output, brain relaxation, urine output, length of stay, and biochemical parameters. More large scale trials should be done with a homogeneous cohort to establish an optimal perioperative fluid management regime in neurosurgical patients.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Al-Ghamdi AA. Intraoperative fluid management: Past and future, where is the evidence?. Saudi J Anaesth. 2018. 12: 311-7
2. Balaka C, Stranjalis G, Kalamatianos T, Koutsarnakis C, Bouras T, Boviatsis E. Perioperative microdialysis in meningioma surgery: Correlation of cerebral metabolites with clinical outcome. Acta Neurochir (Wien). 2014. 12: 25305088
3. Brallier JW, Dalal PJ, McCormick PJ, Lin HM, Deiner SG. Elevated intraoperative serum lactate during craniotomy is associated with new neurological deficit and longer length of stay. J Neurosurg Anesthesiol. 2017. 29: 388-92
4. Cannesson M, Le Manach Y, Hofer CK, Goarin JP, Lehot JJ, Vallet B. Assessing the diagnostic accuracy of pulse pressure variations for the prediction of fluid responsiveness: A “gray zone” approach. Anesthesiology. 2011. 115: 231-41
5. Cecconi M, Corredor C, Arulkumaran N, Abuella G, Ball J, Grounds RM. Clinical review: Goal-directed therapy-what is the evidence in surgical patients? The effect on different risk groups. Crit Care. 2013. 5: 209
6. Chong MA, Wang Y, Berbenetz NM, McConachie I. Does goal-directed haemodynamic and fluid therapy improve peri-operative outcomes?: A systematic review and meta-analysis. Eur J Anaesthesiol. 2018. 35: 469-83
7. Giglio M, Biancofiore G, Corriero A, Romagnoli S, Tritapepe L, Brienza N. Perioperative goal-directed therapy and postoperative complications in different kind of surgical procedures: An updated meta-analysis. J Anesth Analg Crit Care. 2021. 1: 26
8. Gopal J, Srivastava S, Singh N, Haldar R, Verma R, Gupta D. Pulse pressure variance (PPV)-guided fluid management in adult patients undergoing supratentorial tumor surgeries: A randomized controlled trial. Asian J Neurosurg. 2023. 22: 508-15
9. Hand WR, Stoll WD, McEvoy MD, McSwain JR, Sealy CD, Skoner JM. Intraoperative goal-directed hemodynamic management in free tissue transfer for head and neck cancer. Head Neck. 2016. 38: 1974-80
10. Hasanin A, Zanata T, Osman S, Abdelwahab Y, Samer R, Mahmoud M. Pulse pressure variation-guided fluid therapy during supratentorial brain tumour excision: A randomized controlled trial. Open Access Maced J Med Sci. 2019. 10: 2474-9
11. Holte K, Sharrock NE, Kehlet H. Pathophysiology and clinical implications of perioperative fluid excess. Br J Anaesth. 2002. 89: 622-32
12. Hrdy O, Duba M, Dolezelova A, Roskova I, Hlavaty M, Traj R. Effects of goal-directed fluid management guided by a non-invasive device on the incidence of postoperative complications in neurosurgery: A pilot and feasibility randomized controlled trial. Perioper Med (Lond). 2023. 5: 32
13. Jessen MK, Vallentin MF, Holmberg MJ, Bolther M, Hansen FB, Holst JM. Goal-directed haemodynamic therapy during general anaesthesia for noncardiac surgery: A systematic review and meta-analysis. Br J Anaesth. 2022. 128: 416-33
14. Kendrick JB, Kaye AD, Tong Y, Belani K, Urman RD, Hoffman C. Goal-directed fluid therapy in the perioperative setting. J Anaesthesiol Clin Pharmacol. 2019. 35: S29-34
15. Li X, Zhang Q, Zhu Y, Yang Y, Xu W, Zhao Y. Effect of perioperative goal-directed fluid therapy on postoperative complications after thoracic surgery with one-lung ventilation: A systematic review and meta-analysis. World J Surg Oncol. 2023. 18: 297-10
16. Luo J, Xue J, Liu J, Liu B, Liu L, Chen G. Goal-directed fluid restriction during brain surgery: A prospective randomized controlled trial. Ann Intensive Care. 2017. 7: 16
17. Michard F, ChemLa D, Teboul JL. Meta-analysis of pulse pressure variation (PPV) and stroke volume variation (SVV) studies: A few rotten apples can spoil the whole barrel. Crit Care. 2023. 27: 482
18. Mishra N, Rath GP, Bithal PK, Chaturvedi A, Chandra PS, Borkar SA. Effect of goal-directed intraoperative fluid therapy on duration of hospital stay and postoperative complications in patients undergoing excision of large supratentorial tumors. Neurol India. 2022. 70: 108-14
19. Nayak P, Singha SK, Khetrapal M, Sharma A. A randomised controlled study comparing pulse pressure variation (PPV) and Pleth variability index (PVI) for goal-directed fluid therapy intraoperatively in patients undergoing intracranial (supratentorial ICSOLs) surgeries. Rom J Anaesth Intensive Care. 2023. 20: 18-25
20. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ. 2021. 372: n160
21. Pearse RM, Harrison DA, MacDonald N, Gillies MA, Blunt M, Ackland G. Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: A randomized clinical trial and systematic review. JAMA. 2014. 311: 2181-90
22. Peyton PJ, Chong SW. Minimally invasive measurement of cardiac output during surgery and critical care: A meta-analysis of accuracy and precision. Anesthesiology. 2010. 113: 1220-35
23. Sadique Z, Harrison DA, Grieve R, Rowan KM, Pearse RM. Cost-effectiveness of a cardiac output-guided haemodynamic therapy algorithm in high-risk patients undergoing major gastrointestinal surgery. Perioper Med (Lond). 2015. 14: 13
24. Sae-Phua V, Tanasittiboon S, Sangtongjaraskul S. The effect of goal-directed fluid management based on stroke volume variation on ICU length of stay in elderly patients undergoing elective craniotomy: A randomized controlled trial. Indian J Crit Care Med. 2023. 27: 709-16
25. Saugel B, Kouz K, Scheeren TW. The ‘5 Ts’ of perioperative goal-directed haemodynamic therapy. Br J Anaesth. 2019. 123: 103-7
26. Sterne JA, Savović J, Page MJ, Blencowe NS, Boutron I, Cates CJ. RoB 2: A revised tool for assessing the risk of bias in randomised trials. BMJ. 2019. 366: l4898
27. Sundaram SC, Salins SR, Kumar AN, Korula G. Intra-operative fluid management in adult neurosurgical patients undergoing intracranial tumour surgery: Randomised control trial comparing pulse pressure variance (PPV) and central venous pressure (CVP). J Clin Diagn Res. 2016. 10: UCo1-5
28. Wu CY, Lin YS, Tseng HM, Cheng HL, Lee TS, Lin PL. Comparison of two stroke volume variation-based goal-directed fluid therapies for supratentorial brain tumour resection: A randomized controlled trial. Br J Anaesth. 2017. 1: 934942
29. Wu J, Ma Y, Wang T, Xu G, Fan L, Zhang Y. Goal-directed fluid management based on the auto-calibrated arterial pressure-derived stroke volume variation in patients undergoing supratentorial neoplasms surgery. Int J Clin Exp Med. 2017. 10: 3106-14
30. Yildiz GO, Hergunsel GO, Sertcakacilar G, Akyol D, Karakaş S, Cukurova Z. Perioperative goal-directed fluid management using noninvasive hemodynamic monitoring in gynecologic oncology. Braz J Anesthesiol. 2022. 72: 322-30