- Department of Neurosurgery, Federal University of Espirito Santo, Vitoria, Espirito Santo, Brazil
- Department of Geneuro International Research Group in Neuroscience, Vitoria, Brazil
- Department of Neurosurgery Roger Salengro Hospital of Lille University and Regional Hospital Center, Lille, France
- Neurophysiology, Roger Salengro Hospital of Lille University and Regional Hospital Center, Lille, France
Correspondence Address:
Walter Fagundes, Department of Neurosurgery, Federal University of Espirito Santo, Rua Manoel Feu Subtil, Vitoria, Espirito Santo, Brazil.
DOI:10.25259/SNI_1022_2024
Copyright: © 2025 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Walter Fagundes1, Kaike Lobo2, Numa Rajab2, Nicolas Reyns3, Emmanuelle Laureau4, Serge Blond3. Motor cortex stimulation for phantom limb pain treatment. 14-Feb-2025;16:48
How to cite this URL: Walter Fagundes1, Kaike Lobo2, Numa Rajab2, Nicolas Reyns3, Emmanuelle Laureau4, Serge Blond3. Motor cortex stimulation for phantom limb pain treatment. 14-Feb-2025;16:48. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13386
Abstract
Background: Phantom limb pain (PLP) is a chronic neuropathic pain syndrome experienced by individuals following limb amputation. Despite the use of various pharmacological treatments, including opioids, antidepressants, and anticonvulsants, effective pain relief remains challenging for many patients. Motor cortex stimulation (MCS) has emerged as a promising alternative for managing PLP.
Methods: We present the management of three patients with chronic, refractory PLP who underwent epidural MCS at Lille University Hospital Center. The quadripolar electrode lead was implanted into the epidural space under local anesthesia. Stereotactic angiography was used to determine the target coordinates, and the optimal location was confirmed with the guidance of a three-dimensional brain magnetic resonance imaging reconstruction and neurophysiological testing. Pain intensity was assessed using the Visual Analog Scale (VAS) at baseline and at the end of the follow-up period, which had a mean duration of 7 ± 2.16 months.
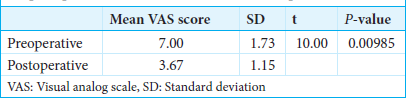
Results: Two of the three patients experienced a decrease in pain by 50%, and one had a 44.4% reduction. The average preoperative VAS score significantly decreased from 7.0 ± 1.73 to 3.67 ± 1.15 at the final follow-up (P = 0.00985). All patients reported a reduction in analgesic medication intake, and no major complications occurred.
Conclusion: PLP is one of the most challenging conditions to treat. MCS is an adjustable and reversible technique that appears to be effective in treating patients with this chronic pain syndrome refractory to other treatment modalities.
Keywords: Motor cortex stimulation, Neuromodulation, Neuropathic pain, Peripheral pain, Phantom limb pain
INTRODUCTION
Phantom limb pain (PLP) is a type of chronic neuropathic pain that arises as a consequence of limb amputation. This condition, primarily affecting individuals with upper or lower extremity amputations, is characterized by the sensation of pain in a limb that no longer exists.[
The earliest description of PLP was described in the 16th century by Ambroise Paré who proposed two neurological models for its cause: peripheral changes in peripheral nerves and cerebral alterations.[
MCS has demonstrated efficacy in reducing chronic neuropathic pain, as evidenced by several clinical trials.[
MATERIALS AND METHODS
Patient population
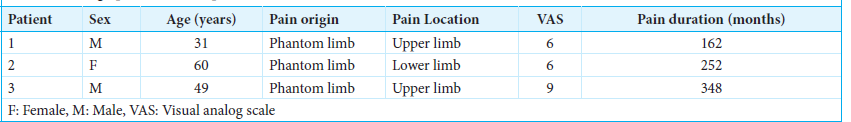
Three patients with chronic PLP were considered eligible for MCS treatment at Lille University Hospital Center. A summary of the patient’s data is presented in
All patients had been treated with various combinations of medications, including antidepressants, anticonvulsants, anti-inflammatory drugs, and opioids. However, these treatments were insufficient to provide adequate pain relief. Before surgery, electrophysiological testing, imaging evaluations, and psychological assessments were conducted for all patients. Individuals displaying significant depressive or neurotic tendencies were excluded as candidates for MCS.
Pain assessment
The pain level and characteristics of each patient were evaluated by a multidisciplinary team at the Pain Clinic associated with our service. Patients were asked to report their pain intensity using a Visual Analog Scale (VAS) at baseline and at the end of the follow-up period, which had a mean duration of 7 ± 2.16 months. Stimulation effects were categorized into four categories: excellent (80–100% pain reduction), good (60–79% reduction), fair (40–59% reduction), and poor (<40% reduction).[
Preoperatively, patients’ VAS scores ranged from 6 to 9, with an average score of 7 ± 1.73. The mean history of pain was 21.16 years.
Surgical procedures
Electrodes were implanted into the epidural space under local anesthesia through a burr hole, following the method originally described by Tsubokawa et al.,[
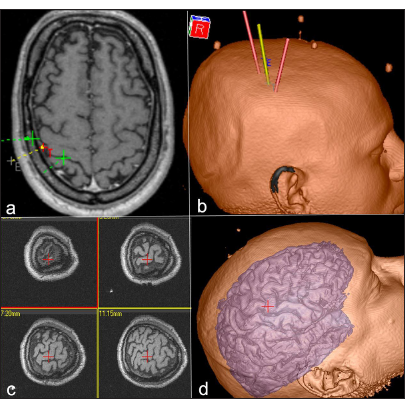
Figure 1:
(a) Axial magnetic resonance imaging (MRI) highlighting the motor cortex (red) and the central sulcus (green) to identify the target site for motor cortex stimulation; (b) 3D reconstruction of the patient’s skull showing the central sulcus projection; (c) Multiplanar views of MRI slices centered on the stimulation site; (d) 3D cortical surface reconstruction emphasizing the operative target for motor cortex stimulation in upper limb pain treatment.
All patients underwent implantation of a quadripolar electrode lead, with each of the 5 mm round electrodes spaced 5 mm apart (Resume™, Medtronic, Inc., Minneapolis, Minnesota). Upon confirming the optimal location using image guidance, electrophysiological testing (including wave inversion N20-P20) [
Postoperative care
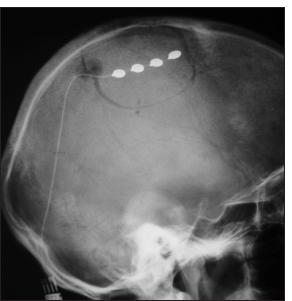
A postoperative skull radiograph was performed to verify the positioning of the electrode array [
A programmer (Medtronic, 7432) was used for the generation and adjustment of stimuli at different parameters by telemetry. Bipolar stimulation was applied using pairs of contacts, while for monopolar stimulation, one contact was designated as the anode or cathode, and the pulse generator served as the opposite pole.
Statistical analysis
Pearson’s Chi-square (χ2) test was applied to analyze parametric data, and Student’s t-test was used for nonparametric variables. All statistical analyses were conducted using Epi-Info 2000™ software (version 6.0, Centers for Disease Control, Atlanta, USA).
RESULTS
Pain relief
Among the three patients with PLP refractory to different therapeutic modalities that epidural MCS treated, two patients experienced a 50% reduction in pain, and one patient experienced a 44.4% reduction. The difference between the mean VAS scores before MCS and at the end of the follow-up period was statistically significant (P = 0.00985) [
In all patients, a reduction in the amount of analgesic medication intake was possible.
Stimulation parameters
Stimulation was initially delivered at a pulse width of 45–60 μs, which increased to 60–210 μs by the final follow-up. The frequency began at 45–60 Hz and was adjusted to 45–130 Hz by the end of treatment. The amplitude initially ranged between 2 and 4 V (mean 2.9 ± 0.57), increasing to 2–5.3 V (mean 4 ± 0.8) over time. The active electrodes were determined through perioperative neurophysiological assessments and adjusted postoperatively based on the patient’s response. Bipolar stimulation was used, with the negative pole positioned over the motor cortex and the positive over the sensory cortex.[
Morbidity
No major complications were observed.
DISCUSSION
PLP is the most prevalent form of postamputation pain syndrome, although its management remains a significant challenge. The pathophysiology underlying PLP involves both peripheral and central nervous system processes. The peripheral mechanisms, in particular, are linked to nerve damage, sensitization from ischemia, and reduced nociceptive thresholds.[
The treatment guidelines generally follow a multidisciplinary approach to the management of pain, with nonsteroidal anti-inflammatory drugs being the most common pharmacological treatment for PLP. Other therapies include opioids, antidepressants, and anticonvulsants. Nonpharmacological therapies, such as transcutaneous electrical nerve stimulation, mirror therapy, and behavioral therapy, are also employed.[
MCS is a nondestructive, adjustable, and reversible technique, making it a preferable option over central neuroablative procedures for managing chronic neuropathic pain [
Positron emission tomography studies have shown that cortical stimulation enhances cerebral blood flow to regions such as the cingulate gyrus, ipsilateral thalamus, orbitofrontal cortex, and brainstem.[
Brodmann area 4 has established connections with the primary and secondary sensory cortices, Brodmann area 5, sensory and motor thalamic nuclei (ventral anterior, ventral lateral, ventral posterolateral, and posterior medial), hypothalamus, periventricular gray matter, and locus coeruleus.[
The rate of patients achieving approximately 50% pain relief in this study aligns with findings from other research, which reported that around half of MCS-treated patients experienced more than 50% reduction in pain.[
It is important to note that even achieving a 40% reduction in pain, while not ideal, represents a meaningful improvement for patients with severe, treatment-resistant pain. Considering the efficacy of other therapeutic approaches, converting intolerable pain into a manageable condition can substantially enhance a patient’s quality of life.
CONCLUSION
PLP is common and one of the most challenging conditions to treat. MCS is an adjustable and reversible technique that appears to be effective in treating patients with this chronic pain syndrome refractory to other treatment modalities.
Ethical approval
The Ethics Committee in Research at the Federal University of São Paulo approved this study on September 9, 2005, under registration number CEP 0969/05.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Brown JA. Motor cortex stimulation. FOC. 2001. 11: 1-5
2. Carroll D, Joint C, Maartens N, Shlugman D, Stein J, Aziz TZ. Motor cortex stimulation for chronic neuropathic pain: A preliminary study of 10 cases. Pain. 2000. 84: 431-7
3. Collins KL, Russell HG, Schumacher PJ, Robinson-Freeman KE, O’Conor EC, Gibney KD. A review of current theories and treatments for phantom limb pain. J Clin Investig. 2018. 128: 2168-76
4. Franzini A, Ferroli P, Servello D, Broggi G. Reversal of thalamic hand syndrome by long-term motor cortex stimulation. J Neurosurg. 2000. 93: 873-5
5. Gunduz ME, Pacheco-Barrios K, Bonin Pinto C, Duarte D, Vélez FG, Gianlorenco AC. Effects of combined and alone transcranial motor cortex stimulation and mirror therapy in phantom limb pain: A randomized factorial trial. Neurorehabil Neural Repair. 2021. 35: 704-16
6. Hamani C, Fonoff ET, Parravano DC, Silva VA, Galhardoni R, Monaco BA. Motor cortex stimulation for chronic neuropathic pain: Results of a double-blind randomized study. Brain. 2021. 144: 2994-3004
7. Katayama Y, Fukaya C, Yamamoto T. Poststroke pain control by chronic motor cortex stimulation: Neurological characteristics predicting a favorable response. J Neurosurg. 1998. 89: 585-91
8. Keil G. “Chose digne d’admiration et quasi incredible”: The “douleur ès parties mortes et amputées”. Fortschr Med. 1990. 108: 62-6
9. Kuffler DP. Evolving techniques for reducing phantom limb pain. Exp Biol Med (Maywood). 2023. 248: 561-72
10. Kuffler DP. Origins of phantom limb pain. Mol Neurobiol. 2018. 55: 60-9
11. Lefaucheur JP, Drouot X, Cunin P, Bruckert R, Lepetit H, Créange A. Motor cortex stimulation for the treatment of refractory peripheral neuropathic pain. Brain. 2009. 132: 1463-71
12. Limakatso K, Ndhlovu F, Usenbo A, Rayamajhi S, Kloppers C, Parker R. The prevalence and risk factors for phantom limb pain: A cross-sectional survey. BMC Neurol. 2024. 24: 57
13. Neil M. Pain after amputation. BJA Educ. 2016. 16: 107-12
14. Nguyen JP, Lefaucheur JP, Decq P, Uchiyama T, Carpentier A, Fontaine D. Chronic motor cortex stimulation in the treatment of central and neuropathic pain. Correlations between clinical, electrophysiological and anatomical data. Pain. 1999. 82: 245-51
15. Nguyen JP, Lefaucheur JP, Le Guerinel C, Eizenbaum JF, Nakano N, Carpentier A. Motor cortex stimulation in the treatment of central and neuropathic pain. Arch Med Res. 2000. 31: 263-5
16. Peyron R, Garcia-Larrea L, Deiber MP, Cinotti L, Convers P, Sindou M. Electrical stimulation of precentral cortical area in the treatment of central pain: Electrophysiological and PET study. Pain. 1995. 62: 275-86
17. Rainov NG, Fels C, Heidecke V, Burkert W. Epidural electrical stimulation of the motor cortex in patients with facial neuralgia. Clin Neurol Neurosurg. 1997. 99: 205-9
18. Raslan AM, McCartney S, Burchiel KJ. Management of chronic severe pain: Cerebral neuromodulatory and neuroablative approaches. Acta Neurochir Suppl. 2007. 97: 17-26
19. Saitoh Y, Hirano S, Kato A, Kishima H, Hirata M, Yamamoto K. Motor cortex stimulation for deafferentation pain. Neurosurg Focus. 2001. 11: E1
20. Saitoh Y, Kato A, Ninomiya H, Baba T, Shibata M, Mashimo T. Primary motor cortex stimulation within the central sulcus for treating deafferentation pain. Acta Neurochir Suppl. 2003. 87: 149-52
21. Saitoh Y, Shibata M, Hirano S, Hirata M, Mashimo T, Yoshimine T. Motor cortex stimulation for central and peripheral deafferentation pain. Report of eight cases. J Neurosurg. 2000. 92: 150-5
22. Schone HR, Baker CI, Katz J, Nikolajsen L, Limakatso K, Flor H. Making sense of phantom limb pain. J Neurol Neurosurg Psychiatry. 2022. 93: 833-43
23. Sherman RA. Published treatments of phantom limb pain. Am J Phys Med. 1980. 59: 232-44
24. Smith H, Joint C, Schlugman D, Nandi D, Stein JF, Aziz TZ. Motor cortex stimulation for neuropathic pain. Neurosurg Focus. 2001. 11: E2
25. Subedi B, Grossberg GT. Phantom limb pain: Mechanisms and treatment approaches. Pain Res Treat. 2011. 2011: 864605
26. Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S. Chronic motor cortex stimulation for the treatment of central pain. Acta Neurochir Suppl (Wien). 1991. 52: 137-9
27. Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S. Chronic motor cortex stimulation in patients with thalamic pain. J Neurosurg. 1993. 78: 393-401