- Department of Neurosurgery, University of Toyama, Toyama, Japan.
Correspondence Address:
Satoshi Kuroda, Department of Neurosurgery, University of Toyama, Toyama, Japan.
DOI:10.25259/SNI_661_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Yuichiro Koga, Daina Kashiwazaki, Emiko Hori, Naoki Akioka, Satoshi Kuroda. Oro-mandibular dystonia in pediatric moyamoya disease: Two cases report. 06-Sep-2021;12:449
How to cite this URL: Yuichiro Koga, Daina Kashiwazaki, Emiko Hori, Naoki Akioka, Satoshi Kuroda. Oro-mandibular dystonia in pediatric moyamoya disease: Two cases report. 06-Sep-2021;12:449. Available from: https://surgicalneurologyint.com/surgicalint-articles/11092/
Abstract
Background: In this report, we describe rare two pediatric cases that developed oro-mandibular dystonia due to moyamoya disease.
Case Description: A 7-year-old boy presented with oro-mandibular dystonia and transient weakness of the left extremities, and was diagnosed as moyamoya disease. Another 7-year-old boy developed oro-mandibular dystonia alone and was diagnosed as moyamoya disease. In both, cerebral blood flow (CBF) was markedly decreased in the involved hemispheres, including the basal ganglia and cerebral cortex. They successfully underwent combined bypass surgery and experienced no further attacks of oromandibular dystonia during follow-up periods. CBF almost normalized through surgical collaterals through direct and indirect bypass.
Conclusion: When treating patients with oro-mandibular dystonia, moyamoya disease should be listed as one of the differential diseases. The underlying mechanism of oro-mandibular dystonia in moyamoya disease is still unclear, but persistent cerebral ischemia in the basal ganglia and/or parietal lobe may play a key role to induce this rare symptom.
Keywords: Bypass surgery, Cerebral blood flow, Involuntary movement, Moyamoya disease, Oromandibular dystonia
INTRODUCTION
Moyamoya disease causes various presentations, but most of the pediatric patients develop transient ischemic attack (TIA) and ischemic stroke.[
This report first describes two pediatric cases of moyamoya disease that manifested involuntary movements in the oro-mandibular area.
CASE DESCRIPTION
Case 1
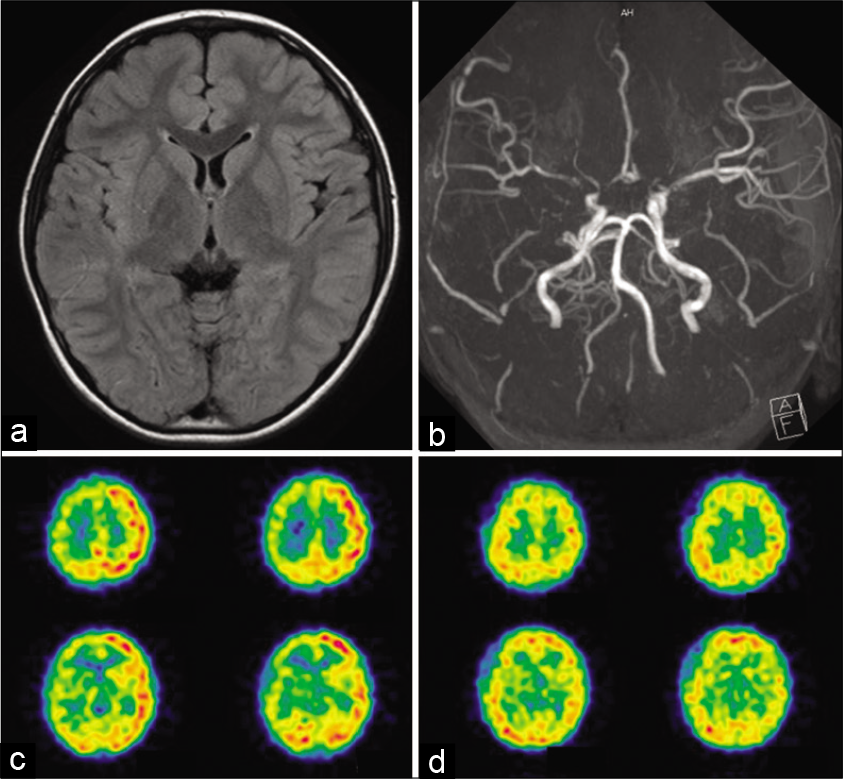
A 7-year-old boy suddenly experienced transient loss of the ability to close his mouth while he was eating. This symptom of jaw opening lasted for about 5 min, followed by transient weakness of the left extremities for a few minutes. Neurological examination on admission revealed no abnormalities. No parenchymal lesions were observed on FLAIR image. MRA and cerebral angiography showed the narrowing of the carotid forks and their branches on both sides. SPECT demonstrated a marked decrease in cerebral blood flow (CBF), including the striatum and cortex, in the right cerebral hemisphere [
Figure 1:
Radiological findings of a 7-year-old boy who developed oro-mandibular dystonia and transient ischemic attack (Case 1). (a) No parenchymal lesions were observed on FLAIR image (b) Magnetic resonance angiography showed the narrowing of the carotid forks and their branches on both sides. Moyamoya vessels were more apparent in the right side. (c) 123I-IMP SPECT demonstrated a marked decrease in cerebral blood flow (CBF) on the right side, including the striatum and cortex. (d) On follow-up SPECT at 4 months after superficial temporal artery to middle cerebral artery anastomosis and encephalo-duro-myo-arteriopericranial synang onto the right side, CBF markedly improved on the operated hemisphere.
Case 2
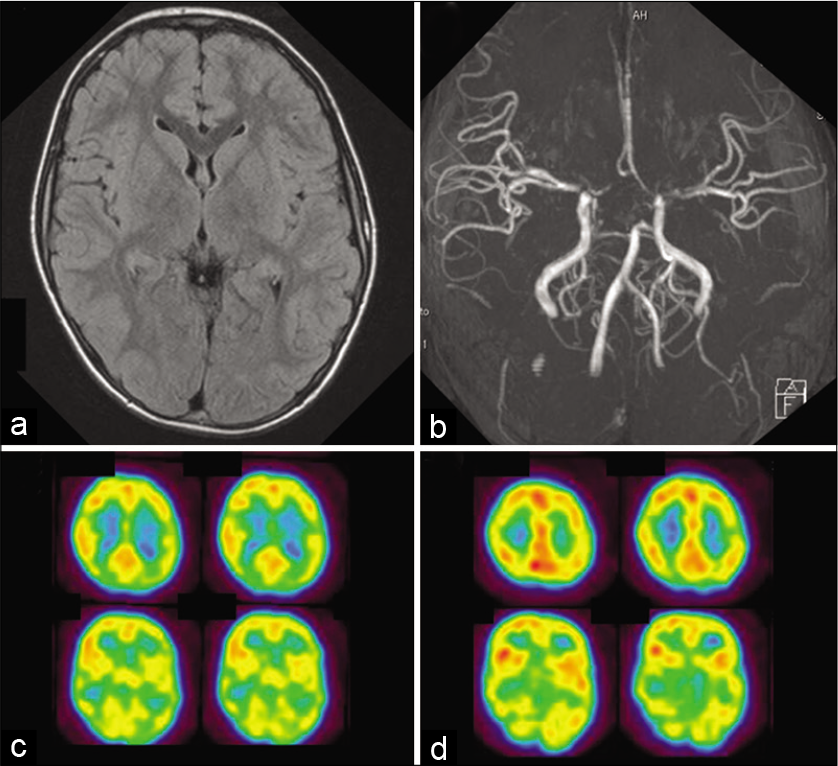
A 7-year-old boy suddenly experienced involuntary movements of the jaw and tongue. Characteristically, dystonic and dyskinetic motions were seen, including jaw opening and the jaw and throat making clicking sounds. Neurological examination on admission showed no abnormalities. No parenchymal lesions were observed on FLAIR image. MRA and cerebral angiography demonstrated severe stenosis of the carotid forks on both sides. SPECT showed a decreased in CBF in the left hemisphere, including the striatum and cortex [
Figure 2:
Radiological findings of a 7-year-old boy who developed oro-mandibular dystonia (Case 2). (a) No parenchymal lesions were observed on FLAIR image (b) Magnetic resonance angiography showed the stenosis of the carotid forks and their branches on both sides. (c) 123I-IMP SPECT demonstrated a significant decrease in cerebral blood flow (CBF) on the left side, including the striatum and cortex. (d) On follow-up SPECT at 6 months after superficial temporal artery to middle cerebral artery anastomosis and encephalo-duro-myo-arterio-pericranial synang on both sides, CBF markedly improved on the operated hemispheres.
DISCUSSION
In the present cases, unique movement disorder around their jaw is considered consistent to oromandibular dystonia. Dystonia is defined as involuntary lasting severe muscle contractions and is defined as oromandibular dystonia when the oral cavity such as the mouth, face, and jaw is involved.[
The mechanisms of involuntary movements in moyamoya disease are still unclear, but it is most likely that the basal ganglia may be largely involved in the occurrence, where the dilated moyamoya vessels are often found.[
Second, the hypertrophied moyamoya vessels in the basal ganglia may physically compress the neurons and/or the extrapyramidal tract in the basal ganglia, causing involuntary movements.[
Finally, involuntary movement may develop through hypermetabolic state in the striatum due to the dilated moyamoya vessels. Thus, glucose metabolism was reported to increase in the striatum responsible for unilateral chorea in two pediatric patients who developed involuntary movements “after” bypass surgery, although they did not experience it before surgery.[
Alternatively, recent studies have shown that dystonia is not only caused by an abnormality of the basal ganglia alone, but also by a functional impairment of other brain regions such as the cerebellum, thalamus, midbrain/brainstem, and cerebral cortex. For example, some investigators have largely focused on the cerebello-thalamo-cortical network.[
CONCLUSION
We report rare two cases that developed oro-mandibular dystonia due to moyamoya disease. The underlying mechanism of oromandibular dystonia in moyamoya disease is still unclear, but persistent cerebral ischemia in the basal ganglia and/or parietal lobe may play a key role in this rare symptom.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Ministry of Health, Labor, and Welfare of Japan.
Conflicts of interest
There are no conflicts of interest.
References
1. Ahn ES, Scott RM, Robertson RL, Smith ER. Chorea in the clinical presentation of moyamoya disease: Results of surgical revascularization and a proposed clinicopathological correlation. J Neurosurg Pediatr. 2013. 11: 313-9
2. Akin A, Yilmaz R, Selcuk F, Akbostanci MC. Sudden onset of oromandibular dystonia after cerebellar stroke. Tremor Other Hyperkinet Mov (NY). 2014. 4: 262
3. Baik JS, Lee MS. Movement disorders associated with moyamoya disease: A report of 4 new cases and a review of literatures. Mov Disord. 2010. 25: 1482-6
4. Burguera JA, Bataller L, Valero C. Action hand dystonia after cortical parietal infarction. Mov Disord. 2001. 16: 1183-5
5. Hoshi E, Tremblay L, Feger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nat Neurosci. 2005. 8: 1491-3
6. Im SH, Oh CW, Kwon OK, Cho BK, Chung YS, Han DH. Involuntary movement induced by cerebral ischemia: Pathogenesis and surgical outcome. J Neurosurg. 2004. 100: 877-82
7. Kuroda S, Houkin K. Moyamoya disease: Current concepts and future perspectives. Lancet Neurol. 2008. 7: 1056-66
8. Kuroda S, Houkin K, Ishikawa T, Nakayama N, Iwasaki Y. Novel bypass surgery for moyamoya disease using pericranial flap: Its impacts on cerebral hemodynamics and long-term outcome. Neurosurgery. 2010. 66: 1093-101
9. Kuroda S, Nakayama N, Yamamoto S, Kashiwazaki D, Uchino H, Saito H. Late (5-20 years) outcomes after STA-MCA anastomosis and encephalo-duro-myo-arteriopericranial synangiosis in patients with moyamoya disease. J Neurosurg. 2020. 134: 909-16
10. LeDoux MS, Lorden JF, Ervin JM. Cerebellectomy eliminates the motor syndrome of the genetically dystonic rat. Exp Neurol. 1993. 120: 302-10
11. Lee JY, Kim SK, Wang KC, Chae JH, Cheon JE, Choi JW. Involuntary movement in pediatric moyamoya disease patients: Consideration of pathogenetic mechanism using neuroimaging studies. Childs Nerv Syst. 2014. 30: 885-90
12. Mink JW. Basal ganglia mechanisms in action selection, plasticity, and dystonia. Eur J Paediatr Neurol. 2018. 22: 225-9
13. Neychev VK, Gross RE, Lehericy S, Hess EJ, Jinnah HA. The functional neuroanatomy of dystonia. Neurobiol Dis. 2011. 42: 185-201
14. Pandey P, Bell-Stephens T, Steinberg GK. Patients with moyamoya disease presenting with movement disorder. J Neurosurg Pediatr. 2010. 6: 559-66
15. Raoofi S, Khorshidi H, Najafi M. Etiology, diagnosis and management of oromandibular dystonia: An update for stomatologists. J Dent (Shiraz). 2017. 18: 73-81
16. Scott RM, Smith JL, Robertson RL, Madsen JR, Soriano SG, Rockoff MA. Long-term outcome in children with moyamoya syndrome after cranial revascularization by pial synangiosis. J Neurosurg. 2004. 100: 142-9
17. Sugita Y, Funaki T, Takahashi JC, Takagi Y, Fushimi Y, Kikuchi T. Reversible striatal hypermetabolism in chorea associated with moyamoya disease: A report of two cases. Childs Nerv Syst. 2016. 32: 2243-7
18. Suzuki J, Takaku A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969. 20: 288-99