- Department of Neurosurgery, Faculty of Medicine, Menoufia University, Shebin Elkom, Egypt
Correspondence Address:
Hosam-Eldin Abd-Elazim Habib, Department of Neurosurgery, Faculty of Medicine, Menoufia University, Shebin Elkom, Egypt.
DOI:10.25259/SNI_363_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hosam-Eldin Abd-Elazim Habib, Hossam Elnoamany, Ahmed Gabry Elnaggar. Subgaleal drain versus dissection of subgaleal space and closure without drain after burr-hole drainage of chronic subdural hematoma. 16-Aug-2024;15:288
How to cite this URL: Hosam-Eldin Abd-Elazim Habib, Hossam Elnoamany, Ahmed Gabry Elnaggar. Subgaleal drain versus dissection of subgaleal space and closure without drain after burr-hole drainage of chronic subdural hematoma. 16-Aug-2024;15:288. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13044
Abstract
Background: Chronic subdural hematoma (CSDH) is a collection of blood, blood degradation products, and fluid that accumulate on the surface of the brain between its arachnoid and dural coverings. This study is to evaluate the efficacy of subgaleal drain (SGD) versus subgaleal dissection without drainage as adjuncts to burr-hole evacuation of CSDH.
Methods: A retrospective study was conducted utilizing the data of 60 patients operated for symptomatic CSDH. Patients were divided into two groups, each thirty consecutive patients: Group I, in which a SGD was inserted after CSDH evacuation through a burr-hole; and Group II, the hematoma was evacuated as in the Group I, but with no SGD insertion but instead a subgaleal pocket was created for drainage.
Results: The neurological improvement at 24 h, discharge, 2 weeks, and 6 months after surgery was comparable in both groups. The overall recurrence was 4 cases (4/60, 6.7%). The rate of recurrence and surgical infection rate were comparable in both groups. Both groups showed similar incidences of postoperative seizures, bleeding, rates of medical complications, and neurological deficits. The overall postoperative mortality was five cases (5/60, 8.3%) with no significant difference between groups.
Conclusion: Blunt dissection to open the subgaleal space and closure without a drain is a safe and efficient alternative to the insertion of a drain after the burr-hole evacuation of CSDH.
Keywords: Chronic subdural, Dissection, Drain, Hematoma, Subgaleal
INTRODUCTION
Chronic subdural hematoma (CSDH) is a collection of blood, blood degradation products, and fluid that accumulate on the surface of the brain between its arachnoid and dural coverings. With the progression of size of CSDH, the brain becomes compressed, leading to neurological sequelae or even brain herniation.[
CSDH was first described in the literature in a report by Johannes Wepfer in 1657 about a patient who died as a result of an “apoplectic event, where he found a large blood-filled cyst under the dura.” In 1857, Virchow implicated inflammation as the major cause of CSDH, and thus, it was termed “pachy meningitis hemorrhagica interna.” Later, trauma was pointed out as the etiology of CSDH by Trotter In 1914. Controversy as regards the origin and natural history of CSDH remained till the end of the 20th century.[
As previously mentioned, cranial trauma accounts for the majority of CSDH patients, where it has been identified in 50–77% of cases.[
The incidence of CSHD increases with age, especially among the elderly; men are more commonly affected than women, and an increase in diagnosis has been observed with improvement and increased availability of computed tomography (CT) scanners.[
CT is the most used diagnostic tool for identification and follow-up of CSDH.[
The subgaleal space lies just deep to the galea and extends from the superior nuchal line posteriorly to the forehead anteriorly and ends laterally, where the galea extends with the temporalis fascia down to the zygomatic arches. This layer is avascular and has absorptive abilities.[
Von Mikulicz conducted the first ventriculosubgaleal shunt (VSGS) in 1896 by dissection of the subgaleal space, allowing the scalp to absorb the excess CSF. It has been utilized for chronic postoperative CSF fistulas, recurring subdural hematoma, tumors, repeated ventriculoperitoneal (VP) shunt infections, and acute head trauma. Numerous institutions favor VSGS because it is a simple and quick technique that eliminates the need for recurrent aspiration without incurring electrolyte and nutritional losses. It is linked with lower rates of infection compared to external ventricular drain due to the lack of external tubes and closed system of CSF drainage.[
In regions where there are insufficient hospital beds and where a rapid turnover is mandatory, it is impossible to discharge the patients with a drain, but it is possible to create a subgaleal pocket that acts as a closed drainage system and provides an absorptive surface. Thus, the purpose of this study is to evaluate to evaluate the efficacy of subgaleal drain (SGD) versus subgaleal dissection without drainage as adjuncts to burr-hole evacuation of CSDH.
MATERIALS AND METHODS
This retrospective study included the data of 60 patients who were operated on for symptomatic CSDH through burr-hole evacuation with and without SGD between January 2017 and January 2023. The study population included two groups; Group I included 30 consecutive patients in which a SGD was inserted after CSDH evacuation through two burr holes; and in Group II, 30 consecutive patients had hematoma evacuation as in Group I, but without SGD insertion, but instead a subgaleal pocket was created for drainage.
Inclusion criteria
The following criteria were included in the study:
Patients aged 18 years or older who were presented with symptomatic CSDH proven by CT scan Patients with subacute (isodense in CT) or acute components (hyperdense components) on top of CSDH.
Exclusion criteria
The following criteria were excluded from the study:
Patients with pure acute or subacute hematoma or extensive membranes necessitating craniotomy CSDH patients with underlying causative conditions (e.g., over drainage of a VP shunt).
On the day of admission, full history taking and neurological assessment were done, brain CT was performed, laboratory investigation (including bleeding and coagulation profile, complete blood picture, renal and hepatic function tests, and blood sugar levels), patients were evaluated for comorbidities, and informed consent was obtained. The severity of the CSDH was graded according to Markwalder’s grading [
Patients were operated on under general anesthesia unless the patients were unfit for general anesthesia local anesthesia, which was then performed. Patients were positioned in a supine position with a headrest. After localizing the maximum width of the hematoma, two separate burr holes, each with a minimum of 14 mm width, were drilled about 7 cm apart. A snip opening of the dura was performed to allow for controlled slow self-drainage of the hematoma, and once the tension was normalized, a cruciate incision of the dura mater was made, and the dural edges were coagulated with bipolar diathermy. The subdural collection was washed out with warm lactated Ringers’ solution.
If a subdural membrane or loculations were found, they were not disrupted except for those under the burr holes. At the end of the procedure, the burr holes were left unsealed to allow free drainage to the subgaleal space. The subdural space was filled with warm lactated Ringers’ solution [
Video 1
In Group I, a 16 gouge catheter drain was inserted in the subgaleal space reaching from the anterior to the posterior burr hole, then tunneled for a minimum of 5 cm away from the scalp incision; the drain was connected to a soft collection bag that was kept in a dependent position for 48 h and then removed [
Figure 1:
(a) Preoperative computed tomography (CT) of Lt frontoparietal chronic subdural hematomas. (b) Intraoperative picture showing the subgaleal drain connected to a collecting bag after surgical evacuation was done with left frontal and parietal burr holes. (c) Postoperative follow-up CT brain; showing good evacuation and resolving of midline shift.
Figure 2:
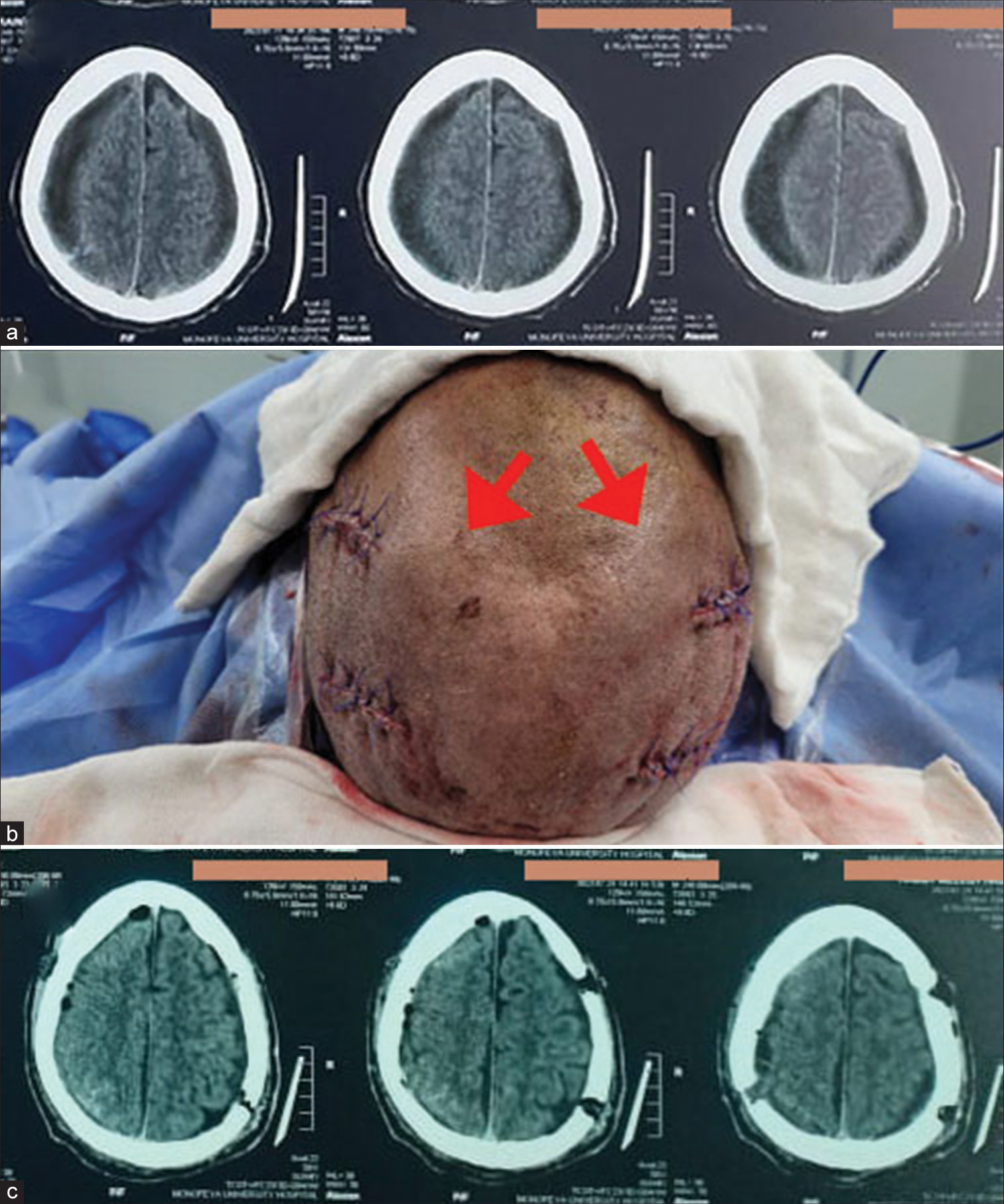
A male patient 68 years old presented with confusion, dysarthria, and quadriparesis. Glasgow coma scale = 13. (a) Preoperative computed tomography (CT) brain revealed bilateral chronic subdural hematomas with bilateral brain compression. (b) Surgical evacuation was done bilaterally at the same time with two burr holes on each side. Subgaleal pockets (red arrows) were created by blunt subgaleal dissection, which was done bilaterally, and closure was done without drain. (c) Postoperative CT brain revealed good decompression.
Video 2
CT brain was performed on the 1st operative day to assess the extent of evacuation and repeated if any neurological deterioration occurred and on discharge. Once the condition of the patients was stationary and not need hospitalization, they were discharged. At discharge, perioperative data, complications, and full neurological assessment (Glasgow coma scale [GCS], mobility, motor power, and speech deficit) were recorded. The Markwalder grading and mRS were recorded at 24 h, at discharge, 2 weeks, and 6 months.
If, within the first 6 postoperative months, the patients had signs and symptoms caused by a subdural hematoma ipsilateral to the side of initial hematoma evacuation, this was considered a recurrence. Re-evacuation of the hematoma was warranted if neurological deficits recurred or progressed.
Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences v26 (IBM Inc., Chicago, IL, USA). Histograms and Shapiro–Wilks confirmed data normality. The two groups were compared using unpaired Student’s t-test for parametric variables’ mean and standard deviation and Mann–Whitney test for non-parametric data’s median and interquartile range. Chi-square or Fisher’s exact tests compared qualitative variables’ frequency and percentage. Two-tailed tests were considered significant when P < 0.05.
RESULTS
The demographic, clinical, and radiological characteristics were comparable between both groups [
The consciousness level was improved postoperatively in 45 cases (45/60, 75%) and regained GCS 14–15 at discharge. The limb weakness was improved postoperatively in all cases that presented with preoperative limb weakness (43/43, 100%) at discharge. The mRS was improved postoperatively to reach 0–3 in 51 cases (51/60, 85%) at discharge. Fifty-two patients (52/60, 86.7%) improved to achieve a Markwalder score of 0–1 at discharge.
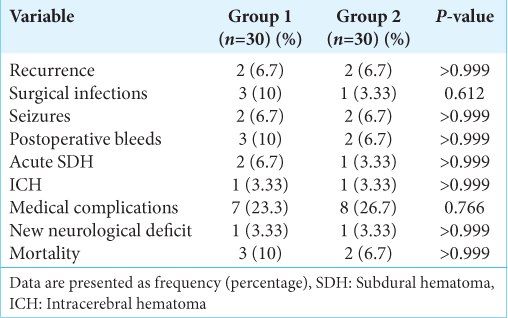
Both groups had similar clinical outcome measures and a similar distribution of patients with neurological improvement at 24 h, discharge, 2 weeks, and 6 months after surgery. There were no statistically significant differences seen in the radiological data at 24 h and 2 weeks post-surgery between the two groups, as shown in
The overall recurrence was 4 cases (4/60, 6.7%). The rate of recurrence and surgical infection rate were comparable in both groups. There was a similar postoperative seizure frequency, postoperative bleeds, medical complications, and new postoperative neurological impairments between groups.
The overall postoperative mortality was 5 cases (5/60, 8.3%) with no significant difference between groups . Three cases in group 1, where a case died due to chest infection, a case due to decompensated liver disease, and the third case developed acute SDH and operated by craniotomy. Two cases in Group 2; one case was due to ischemic heart disease, while the second case was due to acute pulmonary embolism [
DISCUSSION
Chronic subdural hematoma has been managed by different surgical techniques.[
There is no evidence to warrant opening the inner membrane. The inner membrane is highly vascularized and can cause acute hemorrhage. If the hemorrhage occurs away from the surgical site, it may be difficult to control.[
The complications related to treatment are focused mainly on the recurrence post burr-hole evacuation of CSDHs. The rate of CSDH recurrence that requires re-intervention in the present study was comparable in both groups and comparable to that published in the literature, which varies from 9% to 33%.[
Several variables, such as diabetes mellitus, and preoperative hematoma width (>20 mm), preoperative seizure, have been related to the recurrence of CSDH, with the role of antithrombotic drugs, which are still debated.[
Although bilateral CSDH is reported to have a higher recurrence rate that may be attributed to a higher incidence of pneumocephalus or a poor re-expansion of the brain, especially when both sides are done at the same time,[
The use of drainage after burr-hole evacuation of chronic SDH has become debatable. Santarius et al. reported both a reduction in CSDH recurrence and an improvement in the functional outcomes with subdural drain (SDD) insertion.[
Inserting subperiosteal/SGDs after burr-hole drainage of CSDH is an effective and safe substitute to inserting an SDD, with recurrence rates comparable to SDD and significant reduction in the rates of surgical infection, drain misplacements, and the occurrence of iatrogenic brain injuries, suggesting that SGDs can be utilized routinely, and thus, it was implemented in the first group of this study.[
There was neurological improvement and radiological measurements were comparable in the two studied groups. The overall rates of surgical morbidity and mortality were equally distributed between the two studied groups. The hospital stay was slightly shorter in the subgaleal dissection group compared to the SGD group, which needed hospitalization until the drain was removed. The group that operated through burr-hole evacuation without SGD had lower rates of surgical infection than the group that operated through burr-hole evacuation with SGD. Three (10%) cases in the group without SGD had wound infection, as opposed to 1 (3.33%) in the group with SGD. The total recurrences (recollection and acute) in both groups were 23%, of which recurrent CSDH constitutes 6.7 % for each group, which matches the reported rates in literature, which varies from 5% to 30% after surgical evacuation of CSDH.[
CONCLUSION
Blunt dissection to open the subgaleal space and closure without drain is a safe and efficient alternative to insertion of a drain after the burr-hole evacuation of CSDH with comparable recurrence rates and outcome, thus can be utilized in routine clinical practice.
Ethical approval
The research/study approved by the Institutional Review Board at the study was approved by the Local Ethical Scientific Committee of the Menoufia Faculty of Medicine. Menoufia, Egypt. The Institutional Review Board (IRB) approval number 7/2022NEUS9-2, dated 7/2022.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Videos available on:
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Abecassis IJ, Kim LJ. Craniotomy for treatment of chronic subdural hematoma. Neurosurg Clin N Am. 2017. 28: 229-37
2. Achakzai NU, Adil I, Khan S. Outcome of surgical management of chronic subdural hematoma. Pak J Neurol Surg. 2018. 229: 61-6
3. Adhiyaman V, Chattopadhyay I, Irshad F, Curran D, Abraham S. Increasing incidence of chronic subdural haematoma in the elderly. QJM. 2017. 110: 375-8
4. Almenawer SA, Farrokhyar F, Hong C, Alhazzani W, Manoranjan B, Yarascavitch B. Chronic subdural hematoma management: A systematic review and meta-analysis of 34,829 patients. Ann Surg. 2014. 259: 449-57
5. Altaf I, Shams S, Vohra AH. Radiolological predictors of recurrence of chronic subdural hematoma. Pak J Med Sci. 2018. 34: 194-7
6. Castellani RJ, Mojica-Sanchez G, Schwartzbauer G, Hersh DS. Symptomatic acute-on-chronic subdural hematoma: A clinicopathological study. Am J Forensic Med Pathol. 2017. 38: 126-30
7. Chih AN, Hieng AW, Rahman NA, Abdullah JM. Subperiosteal drainage versus subdural drainage in the management of chronic subdural hematoma (A comparative study). Malays J Med Sci. 2017. 24: 21-30
8. Ding H, Liu S, Quan X, Liao S, Liu L. Subperiosteal versus subdural drain after burr hole drainage for chronic subdural hematomas: A systematic review and meta-analysis. World Neurosurg. 2020. 136: 90-100
9. Edlmann E, Giorgi-Coll S, Whitfield PC, Carpenter KL, Hutchinson PJ. Pathophysiology of chronic subdural haematoma: Inflammation, angiogenesis and implications for pharmacotherapy. J Neuroinflammation. 2017. 14: 108
10. Gaist D, Rodríguez LA, Hellfritzsch M, Poulsen FR, Halle B, Hallas J. Association of antithrombotic drug use with subdural hematoma risk. JAMA. 2017. 317: 836-46
11. Guha D, Coyne S, Macdonald RL. Timing of the resumption of antithrombotic agents following surgical evacuation of chronic subdural hematomas: A retrospective cohort study. J Neurosurg. 2016. 124: 750-9
12. Holl DC, Volovici V, Dirven CM, Peul WC, van Kooten F, Jellema K. Pathophysiology and nonsurgical treatment of chronic subdural hematoma: From past to present to future. World Neurosurg. 2018. 116: 402-11.e2
13. Kerr EE, Prevedello DM, Jamshidi A, Ditzel Filho LF, Otto BA, Carrau RL. Immediate complications associated with high-flow cerebrospinal fluid egress during endoscopic endonasal skull base surgery: Report of 2 cases. Neurosurg Focus. 2014. 37: E3
14. Kung WM, Hung KS, Chiu WT, Tsai SH, Lin JW, Wang YC. Quantitative assessment of impaired post-evacuation brain re-expansion in bilateral chronic subdural haematoma: Possible mechanism of the higher recurrence rate. Injury. 2012. 43: 598-602
15. Lega BC, Danish SF, Malhotra NR, Sonnad SS, Stein SC. Choosing the best operation for chronic subdural hematoma: A decision analysis. J Neurosurg. 2010. 113: 615-21
16. Macdonald RL, Winn RH, editors. Pathophysiology of chronic subdural hematoma. Youmans and Winn neurological surgery. Amsterdam: Elsevier; 2017. p.
17. Markwalder TM, Steinsiepe KF, Rohner M, Reichenbach W, Markwalder H. The course of chronic subdural hematomas after burr-hole craniostomy and closed-system drainage. J Neurosurg. 1981. 55: 390-6
18. Mehta V, Harward SC, Sankey EW, Nayar G, Codd PJ. Evidence based diagnosis and management of chronic subdural hematoma: A review of the literature. J Clin Neurosci. 2018. 50: 7-15
19. Miah IP, Tank Y, Rosendaal FR, Peul WC, Dammers R, Lingsma HF. Radiological prognostic factors of chronic subdural hematoma recurrence: A systematic review and meta-analysis. Neuroradiology. 2021. 63: 27-40
20. Motiei-Langroudi R, Stippler M, Shi S, Adeeb N, Gupta R, Griessenauer CJ. Factors predicting reoperation of chronic subdural hematoma following primary surgical evacuation. J Neurosurg. 2017. 129: 1143-50
21. Nassiri F, Hachem LD, Wang JZ, Badhiwala JH, Zadeh G, Gladstone D. Reinitiation of anticoagulation after surgical evacuation of subdural hematomas. World Neurosurg. 2020. 135: e616-22
22. Nouri A, Gondar R, Schaller K, Meling T. Chronic subdural hematoma (cSDH): A review of the current state of the art. Brain Spine. 2021. 1: 100300
23. Perret GE, Graf CJ. Subgaleal shunt for temporary ventricle decompression and subdural drainage. J Neurosurg. 1977. 47: 590-5
24. Rauhala M, Luoto TM, Huhtala H, Iverson GL, Niskakangas T, Öhman J. The incidence of chronic subdural hematomas from 1990 to 2015 in a defined Finnish population. J Neurosurg. 2019. 132: 1147-57
25. Sahyouni R, Mahboubi H, Tran P, Roufail JS, Chen JW. Membranectomy in chronic subdural hematoma: Meta-analysis. World Neurosurg. 2017. 104: 418-29
26. Santarius T, Kirkpatrick PJ, Ganesan D, Chia HL, Jalloh I, Smielewski P. Use of drains versus no drains after burr-hole evacuation of chronic subdural haematoma: A randomised controlled trial. Lancet. 2009. 374: 1067-73
27. Savitz MH, Malis LI. Subgaleal shunting: A 20-year experience. Neurosurg Focus. 2000. 9: ecp1
28. Seery GE. Surgical anatomy of the scalp. Dermatol Surg. 2020. 28: 581-7
29. Soleman J, Lutz K, Schaedelin S, Kamenova M, Guzman R, Mariani L. Subperiosteal vs subdural drain after burr-hole drainage of chronic subdural hematoma: A randomized clinical trial (cSDH-Drain-Trial). Neurosurgery. 2019. 85: E825-34
30. Steinbok P, Cochrane DD. Ventriculosubgaleal shunt in the management of recurrent ventriculoperitoneal shunt infection. Childs Nerv Syst. 1994. 10: 536-9
31. Waqas M, Vakhari K, Weimer PV, Hashmi E, Davies JM, Siddiqui AH. Safety and effectiveness of embolization for chronic subdural hematoma: Systematic review and case series. World Neurosurg. 2019. 126: 228-36
32. Wilson JT, Hareendran A, Hendry A, Potter J, Bone I, Muir KW. Reliability of the modified Rankin Scale across multiple raters: Benefits of a structured interview. Stroke. 2005. 36: 777-81
33. Yadav YR, Parihar V, Chourasia ID, Bajaj J, Namdev H. The role of subgaleal suction drain placement in chronic subdural hematoma evacuation. Asian J Neurosurg. 2016. 11: 214-8